St 11 Introduction to Chemical Reactions Making new


















- Slides: 43

St. 11: Introduction to Chemical Reactions Making new substances

Main Ideas Chemical Reactions are represented by Chemical Equations are balanced to show the same number of atoms of each element on each side. The Law of Conservation of Mass says that atoms won’t be created or destroyed in a chemical reaction. That is why you have to balance chemical equations!

Objectives Level 3 Is able to balance chemical equations. Is able to interpret the 5 types of chemical reactions. Level 2 Is able to identify the number of atoms in a formula Is able to understand the concept of conservation of mass as related to the balancing of chemical equations.

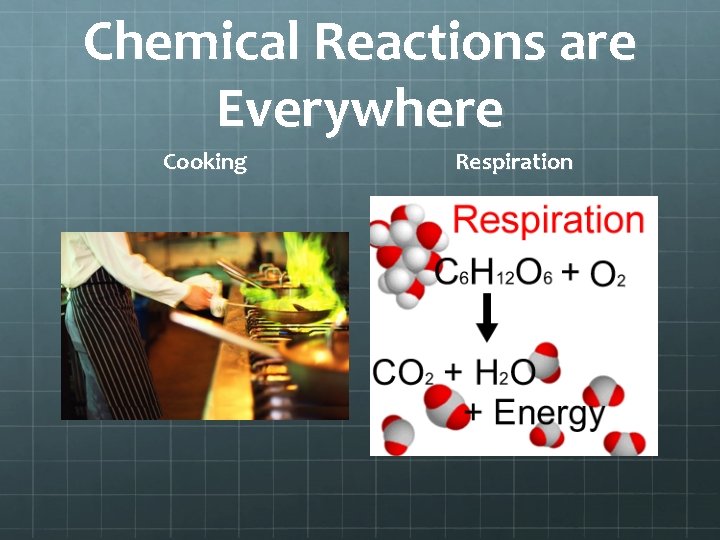
Chemical Reactions are Everywhere Cooking Respiration

Chemical Reactions are Everywhere Hair Dye Auto Fuel

Demo Elephant Toothpaste What do you think is going to happen?

What Happened during Demo? What did you observe during demonstration? Share your observations with someone sitting next to you

How do you know when a chemical reaction takes place? Color Change Precipitate Formation

How do you know when a chemical reaction takes place? Gas Formation Odor

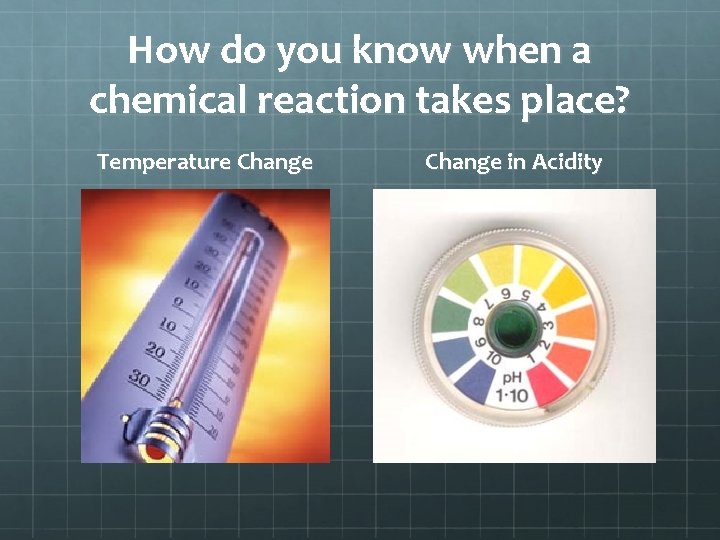
How do you know when a chemical reaction takes place? Temperature Change in Acidity

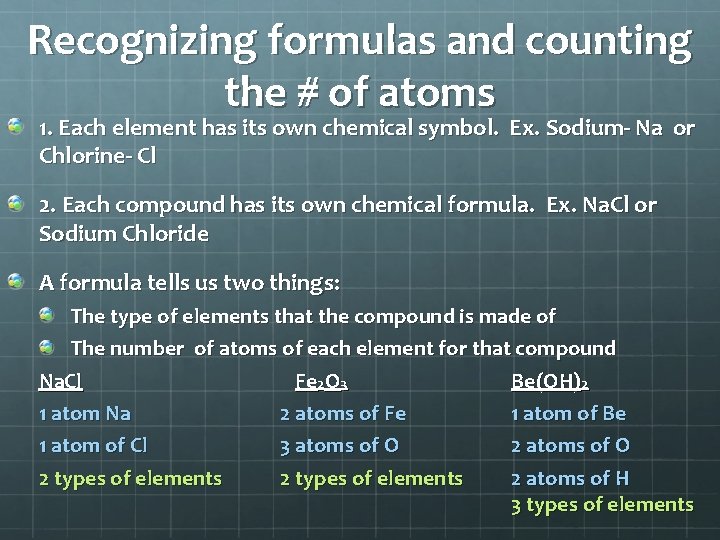
Recognizing formulas and counting the # of atoms 1. Each element has its own chemical symbol. Ex. Sodium- Na or Chlorine- Cl 2. Each compound has its own chemical formula. Ex. Na. Cl or Sodium Chloride A formula tells us two things: The type of elements that the compound is made of The number of atoms of each element for that compound Na. Cl Fe 2 O 3 Be(OH)2 1 atom Na 2 atoms of Fe 1 atom of Be 1 atom of Cl 3 atoms of O 2 types of elements 2 atoms of H 3 types of elements

What if there are parentheses? In the case of parentheses, multiply the subscript throughout the number of atoms that are in the parentheses to get the total number of those atoms. Ex. Be(OH)2 - Take 2 x 1 H and 2 x 1 O, so there are 2 H and 2 O Ex. (NH 4)2 SO 4 - Take 2 x 4 H and 2 times 1 N, so there are 8 H and 2 N

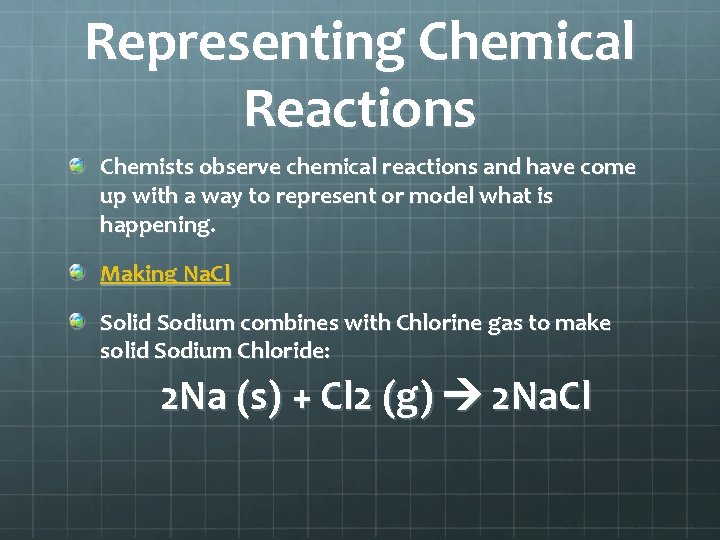
Representing Chemical Reactions Chemists observe chemical reactions and have come up with a way to represent or model what is happening. Making Na. Cl Solid Sodium combines with Chlorine gas to make solid Sodium Chloride: 2 Na (s) + Cl 2 (g) 2 Na. Cl

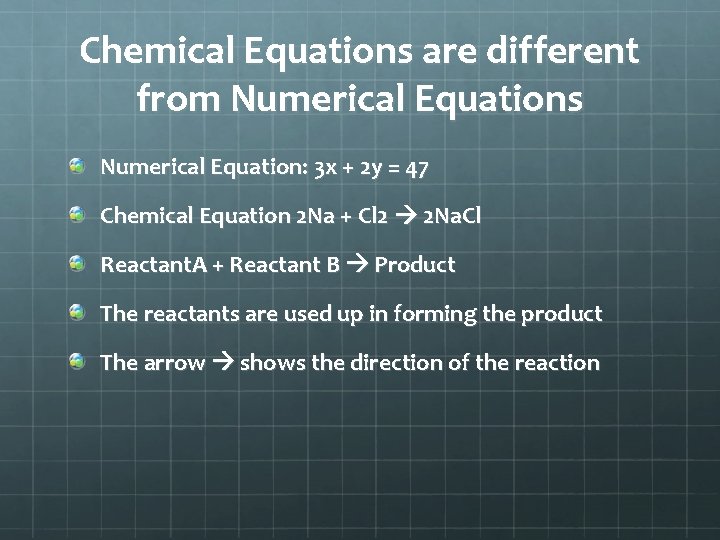
Chemical Equations are different from Numerical Equations Numerical Equation: 3 x + 2 y = 47 Chemical Equation 2 Na + Cl 2 2 Na. Cl Reactant. A + Reactant B Product The reactants are used up in forming the product The arrow shows the direction of the reaction

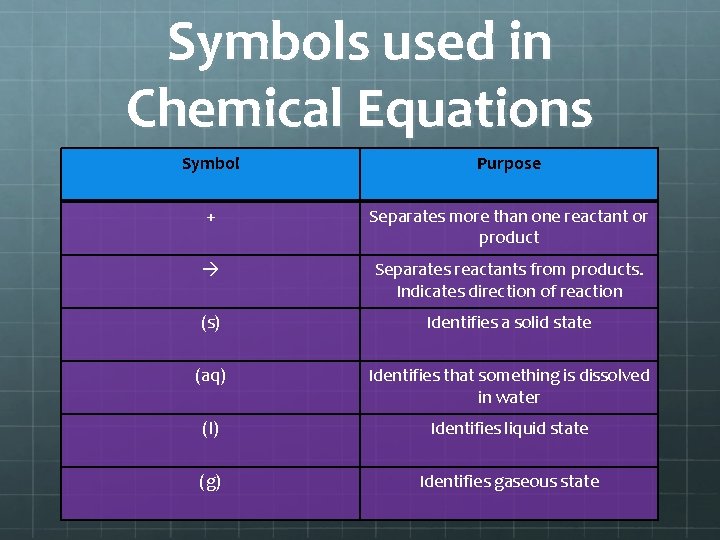
Symbols used in Chemical Equations Symbol Purpose + Separates more than one reactant or product Separates reactants from products. Indicates direction of reaction (s) Identifies a solid state (aq) Identifies that something is dissolved in water (l) Identifies liquid state (g) Identifies gaseous state

Law of Conservation of Mass In a chemical reaction, matter is neither created nor destroyed. Atoms won’t change their identity (e. g. a Carbon atom can’t become an Iron atom) This means that you have to have the same number of each type of atom on each side of the chemical equation. Conservation of Mass Video

Law of Conservation of Mass Video Balancing Equations After you write a chemical equation you have to balance it to make sure that the same number of atoms of each element are on each side. How would you balance this equation? Li + H 2 O H 2 + Li. OH

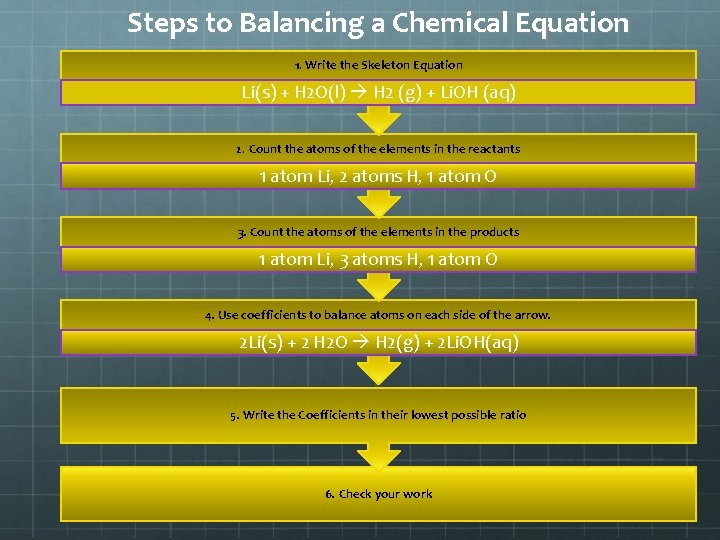
Steps to Balancing a Chemical Equation 1. Write the Skeleton Equation Li(s) + H 2 O(l) H 2 (g) + Li. OH (aq) 2. Count the atoms of the elements in the reactants 1 atom Li, 2 atoms H, 1 atom O 3. Count the atoms of the elements in the products 1 atom Li, 3 atoms H, 1 atom O 4. Use coefficients to balance atoms on each side of the arrow. 2 Li(s) + 2 H 2 O H 2(g) + 2 Li. OH(aq) 5. Write the Coefficients in their lowest possible ratio 6. Check your work

Steps to balancing equations (from previous slide) 1. Write/Read the Skeleton Equation 2. Count the atoms of the elements in the reactants 3. Count the atoms of the elements in the products 4. Use coefficients to balance atoms on each side of the arrow. (the coefficients multiply the # of atoms of the elements in that compound or molecule) 5. Write the Coefficients in their lowest possible ratio 6. Check your work

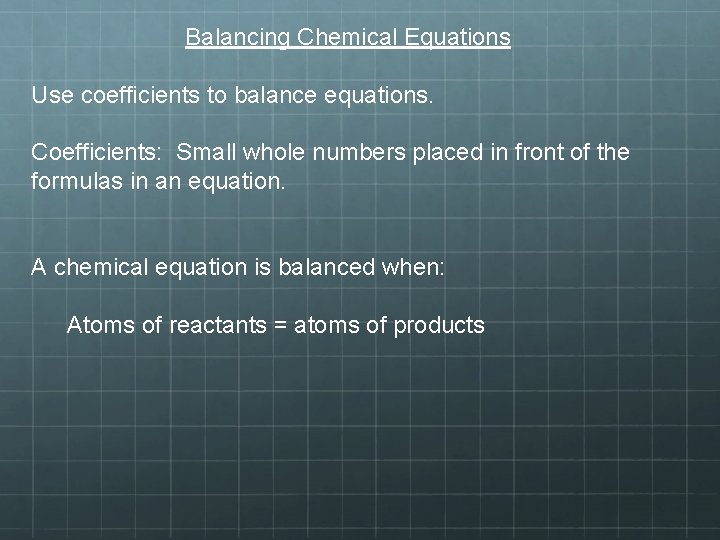
Balancing Chemical Equations Use coefficients to balance equations. Coefficients: Small whole numbers placed in front of the formulas in an equation. A chemical equation is balanced when: Atoms of reactants = atoms of products

Another Example CH 4 (methane gas) + O 2 CO 2 + H 2 O Reactants Products # of Carbons = 1 # of Hydrogens = 4 # of Hydrogens = 2 # of Oxygens = 3 Total atoms = 7 Total atoms = 6 7 ≠ 6! Where did our atoms go?

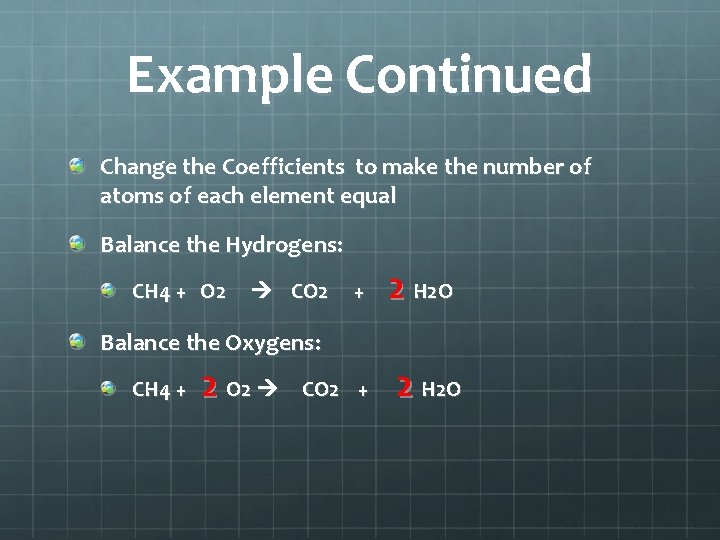
Example Continued Change the Coefficients to make the number of atoms of each element equal Balance the Hydrogens: CH 4 + O 2 CO 2 + 2 H 2 O Balance the Oxygens: CH 4 + 2 O 2 CO 2 + 2 H 2 O

Example Continued CH 4 + 2 O 2 CO 2 + 2 H 2 O Are your coefficients in their simplest ratio? Count your atoms again to check your work: Reactants Products # of Carbons = 1 # of Hydrogens = 4 # of Oxygens = 4 Total atoms = 9

Try These! C 2 H 6 + O 2 CO 2 + H 2 O Fe 2 O 3 + H 2 SO 4 Fe 2(SO 4)3 + H 2 O Hint : balance the polyatomic ion first! Ca. Cl 2 + Ag. NO 3 Ag. Cl + Ca(NO 3)2 Think – Pair - Share

Again, be sure you know: How many atoms? 2 Ca 3(PO 4)2

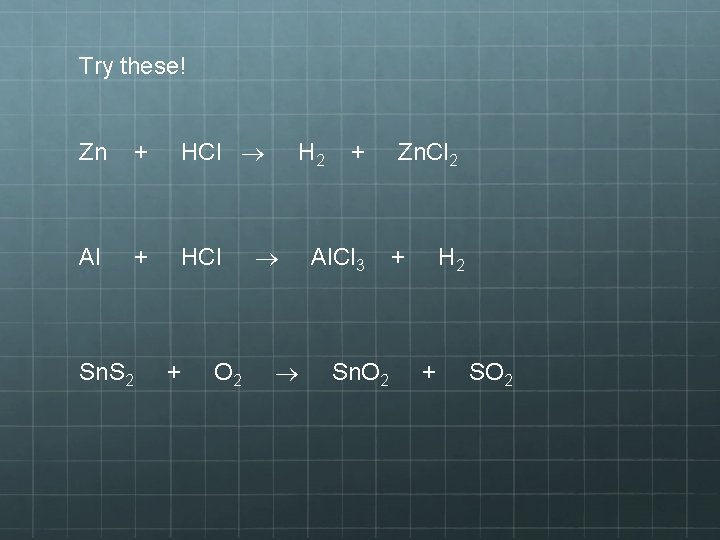
Try these! Zn + HCl Al + HCl Sn. S 2 + O 2 H 2 + Al. Cl 3 Sn. O 2 Zn. Cl 2 + H 2 + SO 2

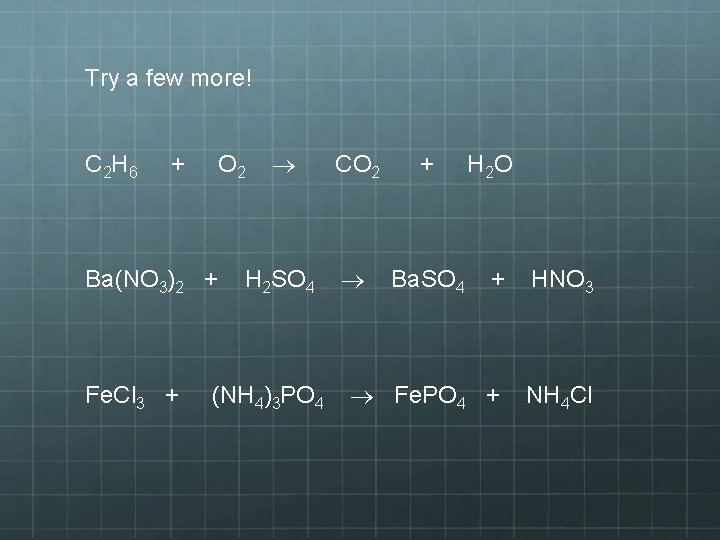
Try a few more! C 2 H 6 + Ba(NO 3)2 + Fe. Cl 3 + CO 2 + H 2 SO 4 Ba. SO 4 O 2 (NH 4)3 PO 4 H 2 O + HNO 3 Fe. PO 4 + NH 4 Cl

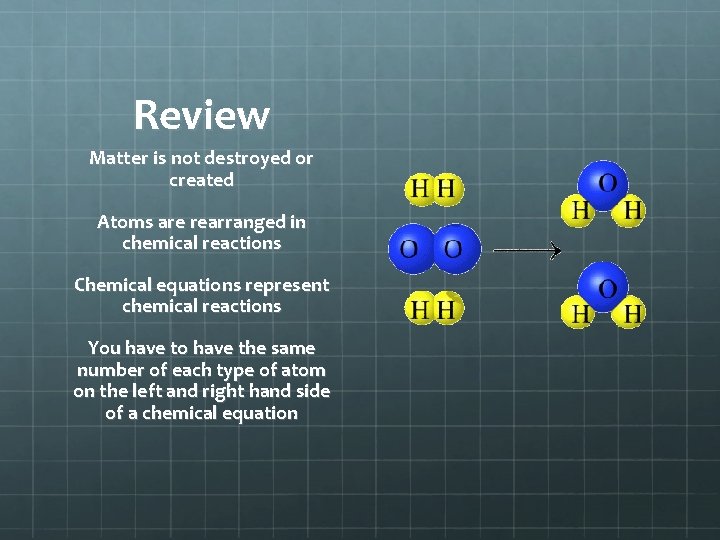
Review Matter is not destroyed or created Atoms are rearranged in chemical reactions Chemical equations represent chemical reactions You have to have the same number of each type of atom on the left and right hand side of a chemical equation

WARNING! Don’t mess with the insides of polyatomic ions – put a square around them, or label them as X – treat the WHOLE polyatomic ion as though it were an element! Don’t ever play around with subscripts (those little numbers that tell you how many atoms are in a molecule) e. g. C 6 H 22 O 11

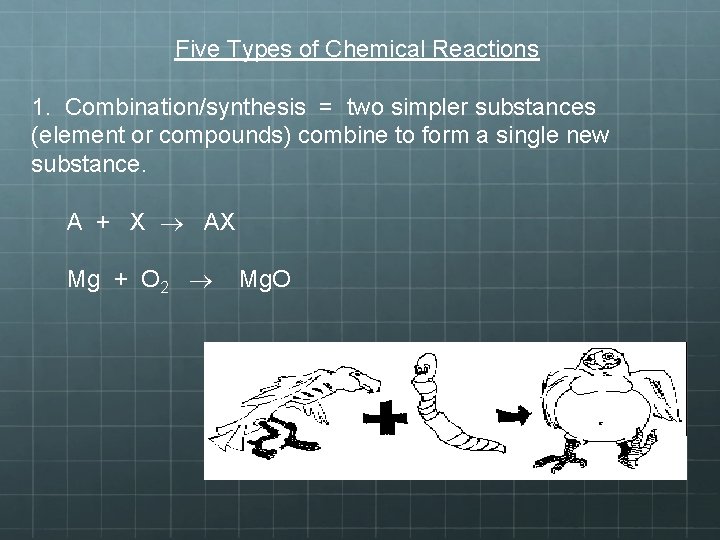
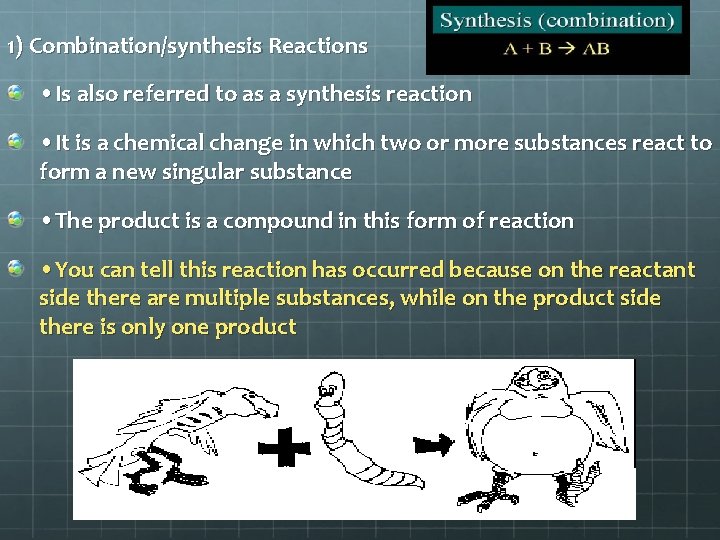
Five Types of Chemical Reactions 1. Combination/synthesis = two simpler substances (element or compounds) combine to form a single new substance. A + X AX Mg + O 2 Mg. O

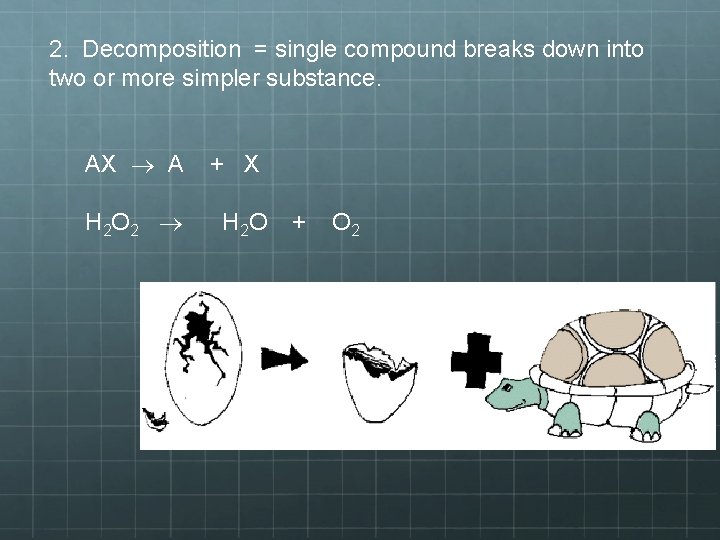
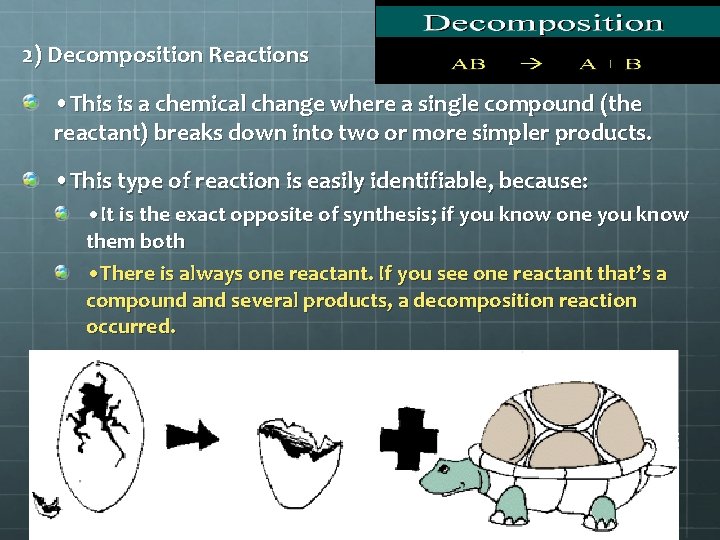
2. Decomposition = single compound breaks down into two or more simpler substance. AX A H 2 O 2 + X H 2 O + O 2

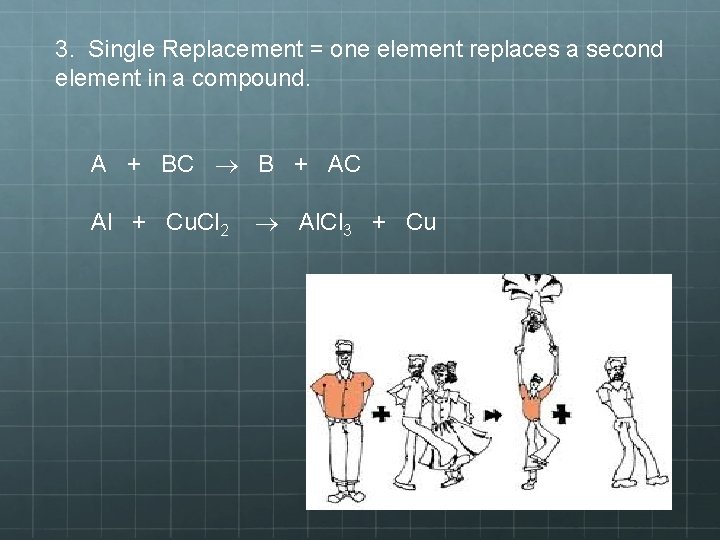
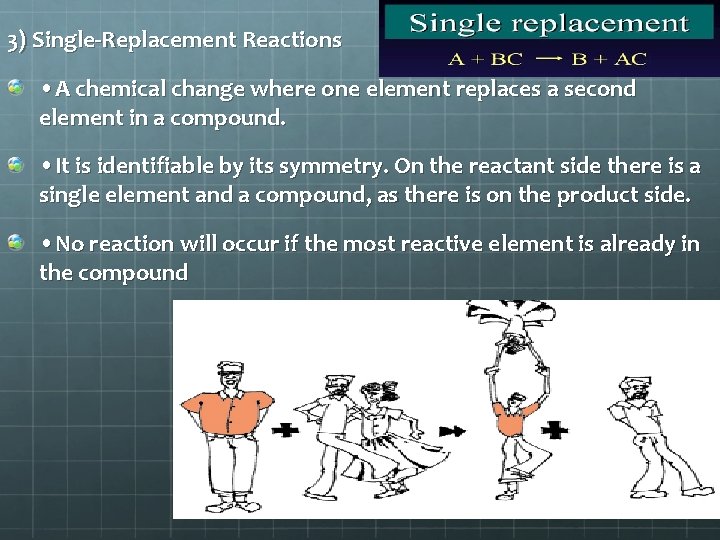
3. Single Replacement = one element replaces a second element in a compound. A + BC B + AC Al + Cu. Cl 2 Al. Cl 3 + Cu

Activity Series of Metals: Lists metals in order of decreasing reactivity. A reactive metal will replace any metal listed below it in the activity series.

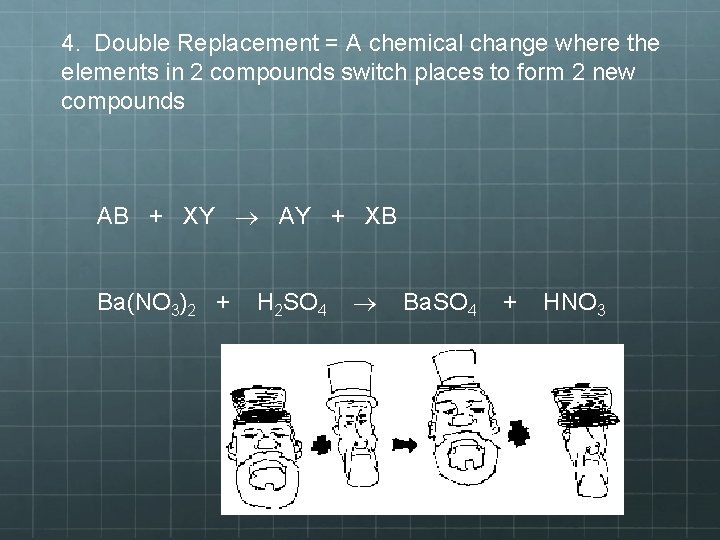
4. Double Replacement = A chemical change where the elements in 2 compounds switch places to form 2 new compounds AB + XY AY + XB Ba(NO 3)2 + H 2 SO 4 Ba. SO 4 + HNO 3

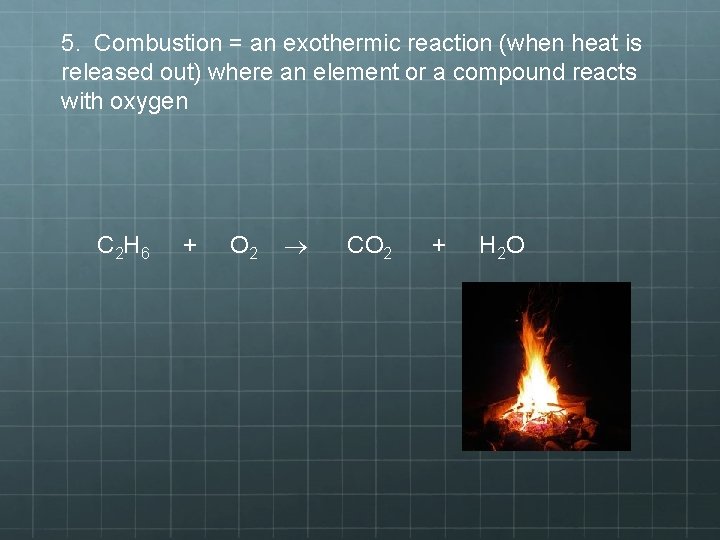
5. Combustion = an exothermic reaction (when heat is released out) where an element or a compound reacts with oxygen C 2 H 6 + O 2 CO 2 + H 2 O

1) Combination/synthesis Reactions • Is also referred to as a synthesis reaction • It is a chemical change in which two or more substances react to form a new singular substance • The product is a compound in this form of reaction • You can tell this reaction has occurred because on the reactant side there are multiple substances, while on the product side there is only one product

2) Decomposition Reactions • This is a chemical change where a single compound (the reactant) breaks down into two or more simpler products. • This type of reaction is easily identifiable, because: • It is the exact opposite of synthesis; if you know one you know them both • There is always one reactant. If you see one reactant that’s a compound and several products, a decomposition reaction occurred.

3) Single-Replacement Reactions • A chemical change where one element replaces a second element in a compound. • It is identifiable by its symmetry. On the reactant side there is a single element and a compound, as there is on the product side. • No reaction will occur if the most reactive element is already in the compound

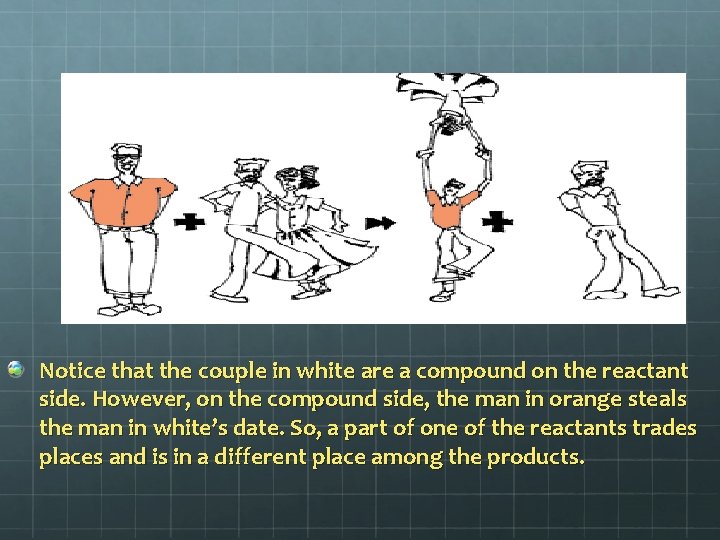
Notice that the couple in white are a compound on the reactant side. However, on the compound side, the man in orange steals the man in white’s date. So, a part of one of the reactants trades places and is in a different place among the products.

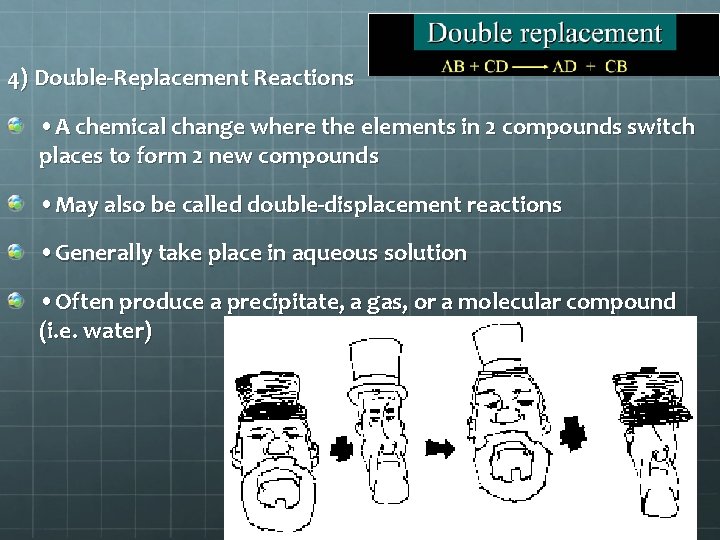
4) Double-Replacement Reactions • A chemical change where the elements in 2 compounds switch places to form 2 new compounds • May also be called double-displacement reactions • Generally take place in aqueous solution • Often produce a precipitate, a gas, or a molecular compound (i. e. water)

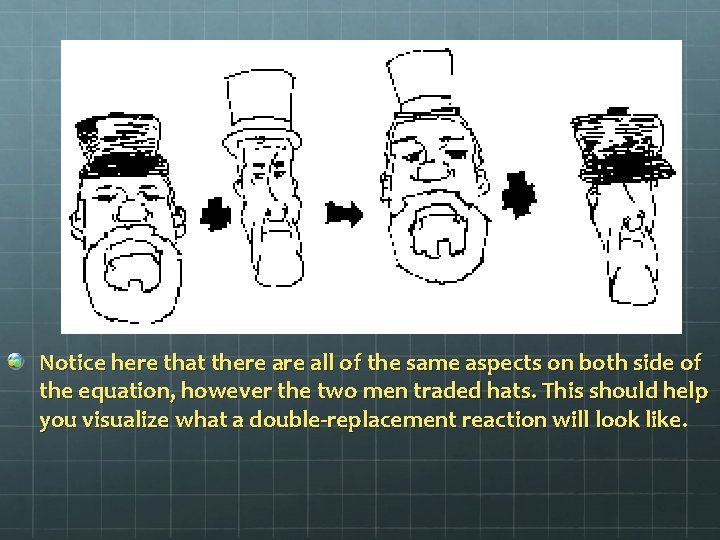
Notice here that there all of the same aspects on both side of the equation, however the two men traded hats. This should help you visualize what a double-replacement reaction will look like.

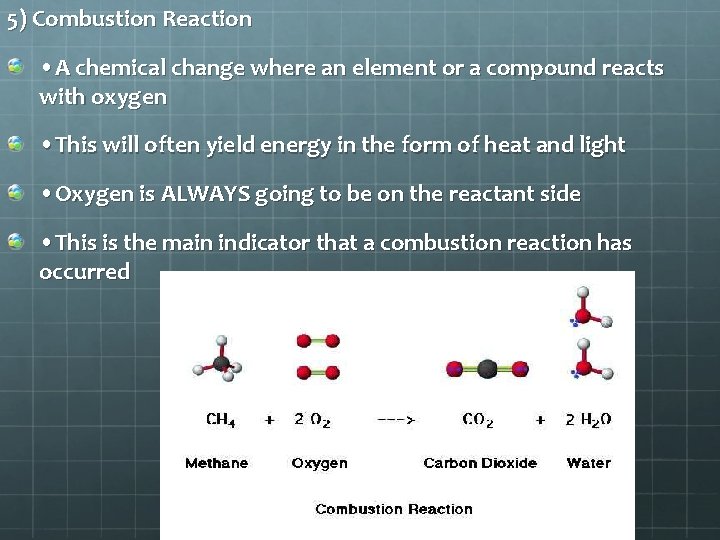
5) Combustion Reaction • A chemical change where an element or a compound reacts with oxygen • This will often yield energy in the form of heat and light • Oxygen is ALWAYS going to be on the reactant side • This is the main indicator that a combustion reaction has occurred
