SPSECAOTs1 Catalytic Wet Peroxide Oxidation CWPO activated by

(SP-SEC-AOTs-1) Catalytic Wet Peroxide Oxidation (CWPO) activated by lanthanum-perovskites of iron copper or manganese L. A. Galeano*, J. C. Delgado E-mail*: alejandrogaleano@udenar. edu. co The First Latin American International Conference on Semiconductor Photocatalysis, Solar Energy Conversion, & Advanced Oxidation Technologies 1 Cartagena, Colombia. May 28 -31, 2013

Outline • Introduction (AOPs; CWPO; Perovskites) • Materials and Methods • Results • Conclusions • Acknowledgments 2

Introduction 3
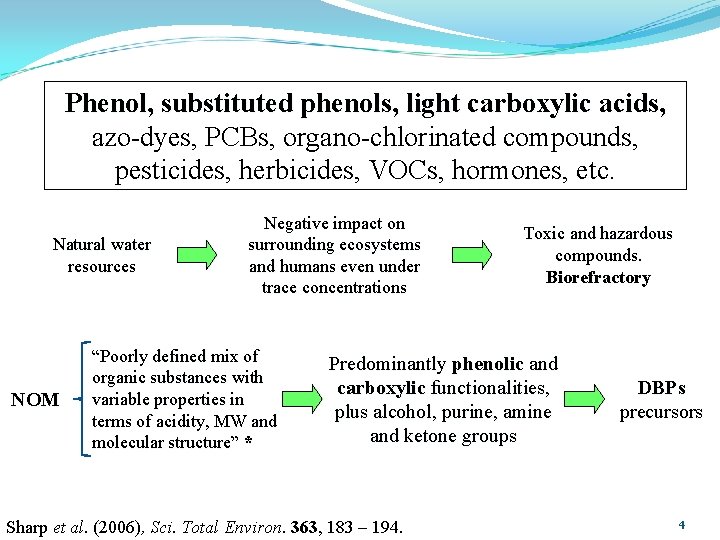
Phenol, substituted phenols, light carboxylic acids, azo-dyes, PCBs, organo-chlorinated compounds, pesticides, herbicides, VOCs, hormones, etc. Natural water resources NOM Negative impact on surrounding ecosystems and humans even under trace concentrations “Poorly defined mix of organic substances with variable properties in terms of acidity, MW and molecular structure” * Toxic and hazardous compounds. Biorefractory Predominantly phenolic and carboxylic functionalities, plus alcohol, purine, amine and ketone groups Sharp et al. (2006), Sci. Total Environ. 363, 183 – 194. DBPs precursors 4
Martínez-Huitle et al. (2009), Appl. Catal. B: Environ. 87, 105 – 145. 5
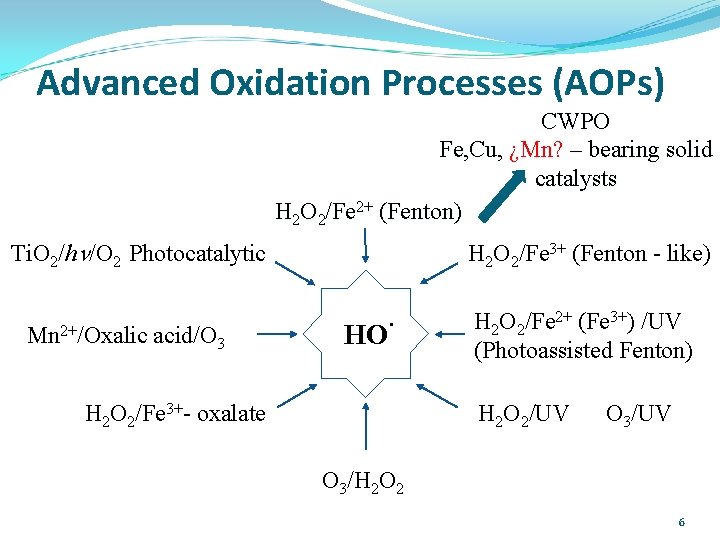
Advanced Oxidation Processes (AOPs) CWPO Fe, Cu, ¿Mn? – bearing solid catalysts H 2 O 2/Fe 2+ (Fenton) Ti. O 2/hn/O 2 Photocatalytic Mn 2+/Oxalic acid/O 3 H 2 O 2/Fe 3+ (Fenton - like) HO· H 2 O 2/Fe 3+- oxalate H 2 O 2/Fe 2+ (Fe 3+) /UV (Photoassisted Fenton) H 2 O 2/UV O 3/H 2 O 2 6
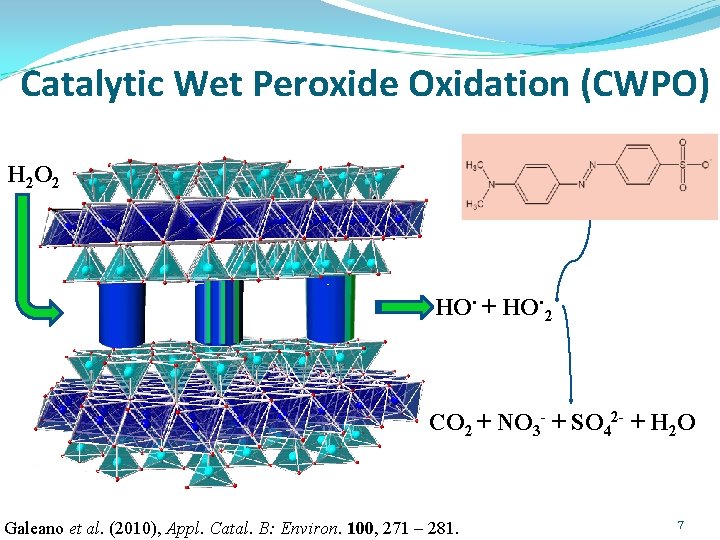
Catalytic Wet Peroxide Oxidation (CWPO) H 2 O 2 HO. + HO. 2 CO 2 + NO 3 - + SO 42 - + H 2 O Galeano et al. (2010), Appl. Catal. B: Environ. 100, 271 – 281. 7
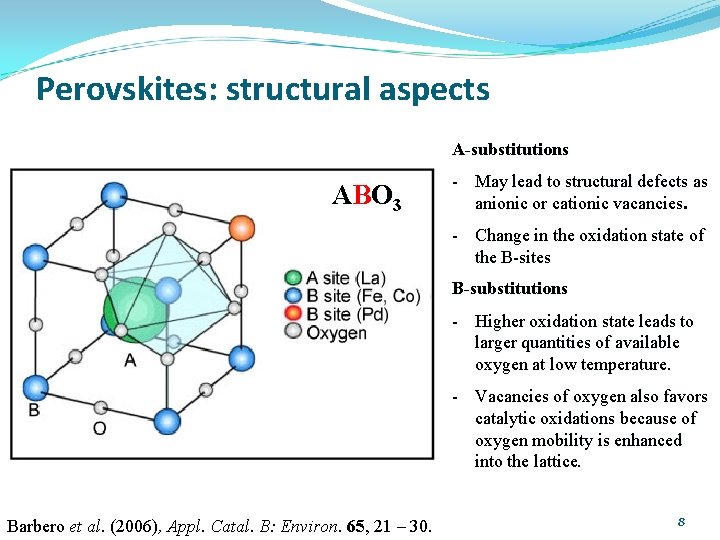
Perovskites: structural aspects A-substitutions AB O 3 - May lead to structural defects as anionic or cationic vacancies. - Change in the oxidation state of the B-sites B-substitutions - Higher oxidation state leads to larger quantities of available oxygen at low temperature. - Vacancies of oxygen also favors catalytic oxidations because of oxygen mobility is enhanced into the lattice. Barbero et al. (2006), Appl. Catal. B: Environ. 65, 21 – 30. 8

Materials and methods 9
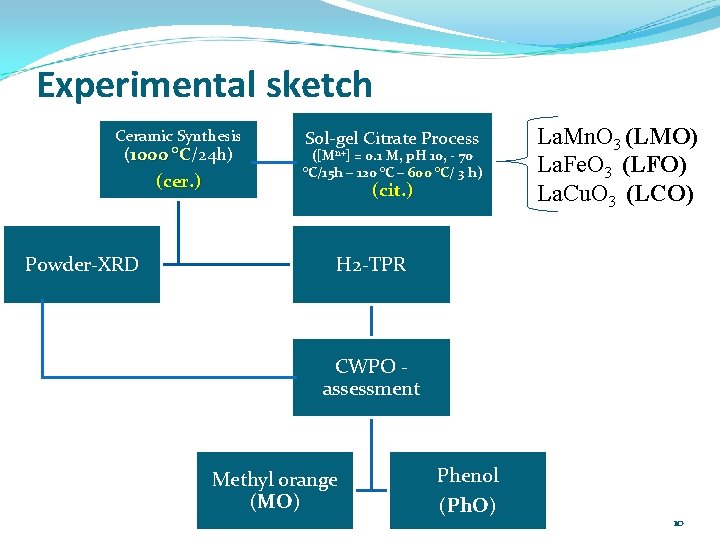
Experimental sketch Ceramic Synthesis (1000 °C/24 h) °C (cer. ) Powder-XRD Sol-gel Citrate Process ([Mn+] = 0. 1 M, p. H 10, - 70 °C/15 h – 120 °C – 600 °C/ °C 3 h) (cit. ) La. Mn. O 3 (LMO) La. Fe. O 3 (LFO) La. Cu. O 3 (LCO) H 2 -TPR CWPO assessment Methyl orange (MO) Phenol (Ph. O) 10
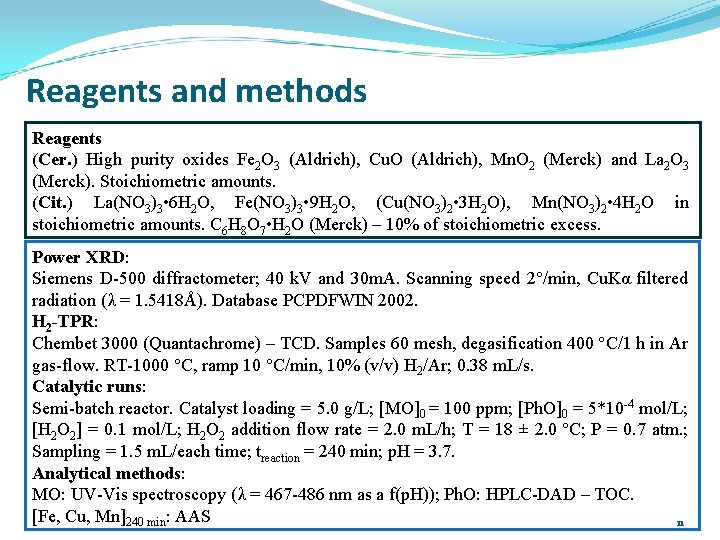
Reagents and methods Reagents (Cer. ) High purity oxides Fe 2 O 3 (Aldrich), Cu. O (Aldrich), Mn. O 2 (Merck) and La 2 O 3 (Merck). Stoichiometric amounts. (Cit. ) La(NO 3)3 • 6 H 2 O, Fe(NO 3)3 • 9 H 2 O, (Cu(NO 3)2 • 3 H 2 O), Mn(NO 3)2 • 4 H 2 O in stoichiometric amounts. C 6 H 8 O 7 • H 2 O (Merck) – 10% of stoichiometric excess. Power XRD: XRD Siemens D-500 diffractometer; 40 k. V and 30 m. A. Scanning speed 2°/min, Cu. Kα filtered radiation (λ = 1. 5418Å). Database PCPDFWIN 2002. H 2 -TPR: -TPR Chembet 3000 (Quantachrome) – TCD. Samples 60 mesh, degasification 400 °C/1 h in Ar gas-flow. RT-1000 °C, ramp 10 °C/min, 10% (v/v) H 2/Ar; 0. 38 m. L/s. Catalytic runs: runs Semi-batch reactor. Catalyst loading = 5. 0 g/L; [MO]0 = 100 ppm; [Ph. O]0 = 5*10 -4 mol/L; [H 2 O 2] = 0. 1 mol/L; H 2 O 2 addition flow rate = 2. 0 m. L/h; T = 18 ± 2. 0 ºC; P = 0. 7 atm. ; Sampling = 1. 5 m. L/each time; treaction = 240 min; p. H = 3. 7. Analytical methods: methods MO: UV-Vis spectroscopy (λ = 467 -486 nm as a f(p. H)); Ph. O: HPLC-DAD – TOC. [Fe, Cu, Mn]240 min: AAS 11

Results 12
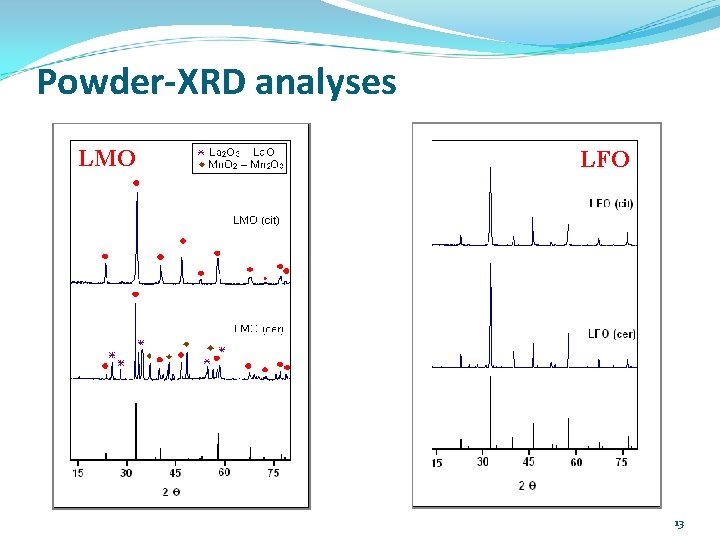
Powder-XRD analyses LMO LFO 13
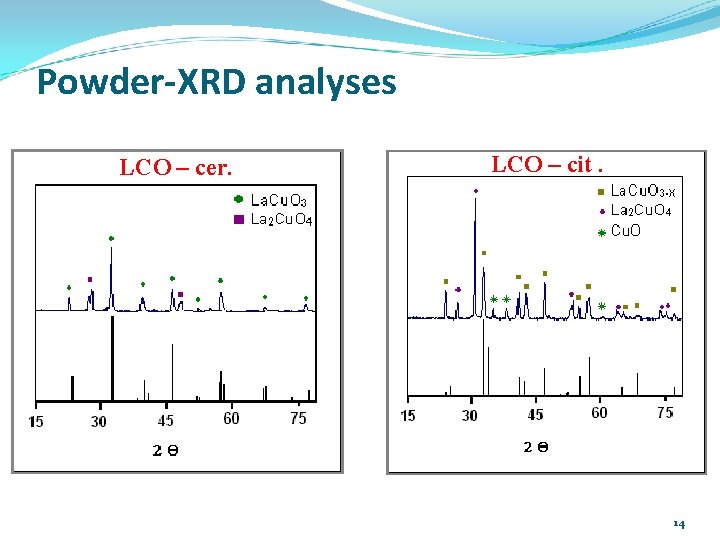
Powder-XRD analyses LCO – cer. LCO – cit. 14
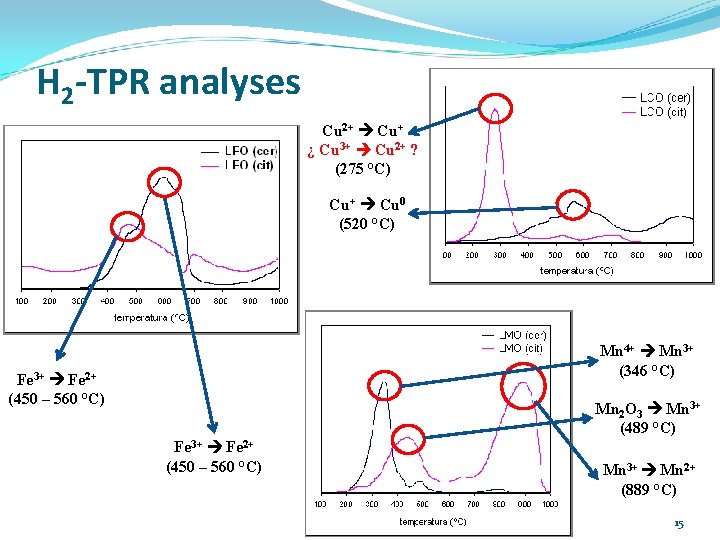
H 2 -TPR analyses Cu 2+ Cu+ ¿ Cu 3+ Cu 2+ ? (275 °C) Cu+ Cu 0 (520 °C) Mn 4+ Mn 3+ (346 °C) Fe 3+ Fe 2+ (450 – 560 °C) Mn 2 O 3 Mn 3+ (489 °C) Fe 3+ Fe 2+ (450 – 560 °C) Mn 3+ Mn 2+ (889 °C) 15
![Catalytic performance: MO removal [Mn]leached = 0. 09 mg/L [Mn]leached = 8. 01 mg/L. Catalytic performance: MO removal [Mn]leached = 0. 09 mg/L [Mn]leached = 8. 01 mg/L.](http://slidetodoc.com/presentation_image_h/9da086361111f2367bae4675da1ac6a5/image-16.jpg)
Catalytic performance: MO removal [Mn]leached = 0. 09 mg/L [Mn]leached = 8. 01 mg/L. p. H = 7. 0 [Cu]leached = 1. 0 mg/L [Cu]leached = 0. 3 mg/L p. H = 5. 5 RT = 18 ºC; P = 0. 7 atm; [MO]0 = 100 mg/L; [catal. ] = 5. 0 g/L; [H 2 O 2]dose = 0. 95 the stoichiometric for full mineralization; [H 2 O 2]flow-rate = 2. 0 m. L/h 16
![Catalytic performance: Ph. O removal [Fe]leached = 0. 7 mg/L [Fe]leached = 0. 3 Catalytic performance: Ph. O removal [Fe]leached = 0. 7 mg/L [Fe]leached = 0. 3](http://slidetodoc.com/presentation_image_h/9da086361111f2367bae4675da1ac6a5/image-17.jpg)
Catalytic performance: Ph. O removal [Fe]leached = 0. 7 mg/L [Fe]leached = 0. 3 mg/L. p. H = 3. 7 RT = 16 ºC; P = 0. 7 atm; [Ph. O]0 = 36 mg C/L; [catal. ] = 5. 0 g/L; [H 2 O 2]dose = 1. 16 the stoichiometric for full mineralization; [H 2 O 2]flow-rate = 2. 0 m. L/h 17
Conclusions Mn and Fe-based La-perovskites can be prepared both pure and well crystallized by the citrate method at T as low as 600 °C. Cu-based perovskites did not properly crystallized even under 1000 °C/24 h, probably because Cu 3+ demands for higher P(O 2) and T. La. Mn. O 3 -(cit. ) showed interesting catalytic behaviour in the CWPO- degradation of MO (73 % of decoulorization at t = 4 h; p. H = 7. 0). 7. 0 La. Fe. O 3 exhibited the higher catalytic potential in the CWPO- degradation of Ph. O (full depletion at t < 1 h; 65 % of TOC mineralized at t = 4 h), comparable to that usually displayed by Al/Fe -PILCs. 18

Acknowledgement Research Group ESCA, UNAL – Bogotá: H 2 -TPR and Ph. O degradation experiments. Prof. M. A. Vicente, University of Salamanca, Spain. XRD analyses. ¡Thank you! 19
- Slides: 19