Spring 2008 Free Radical Copolymerization Radical copolymerization Regular



- Slides: 34

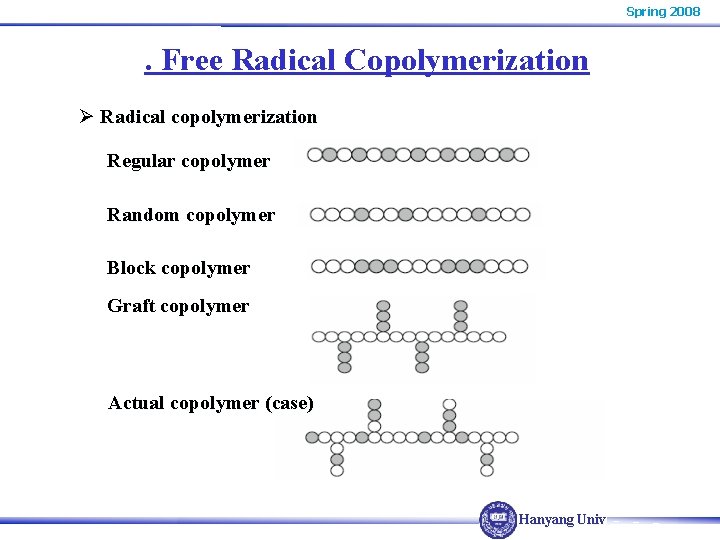
Spring 2008 . Free Radical Copolymerization Ø Radical copolymerization Regular copolymer Random copolymer Block copolymer Graft copolymer Actual copolymer (case) Hanyang Univ.

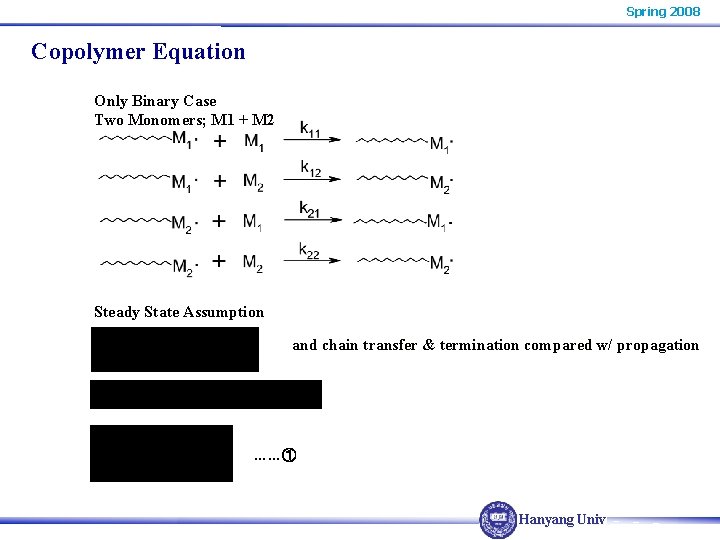
Spring 2008 Copolymer Equation Only Binary Case Two Monomers; M 1 + M 2 Steady State Assumption and chain transfer & termination compared w/ propagation ……① Hanyang Univ.

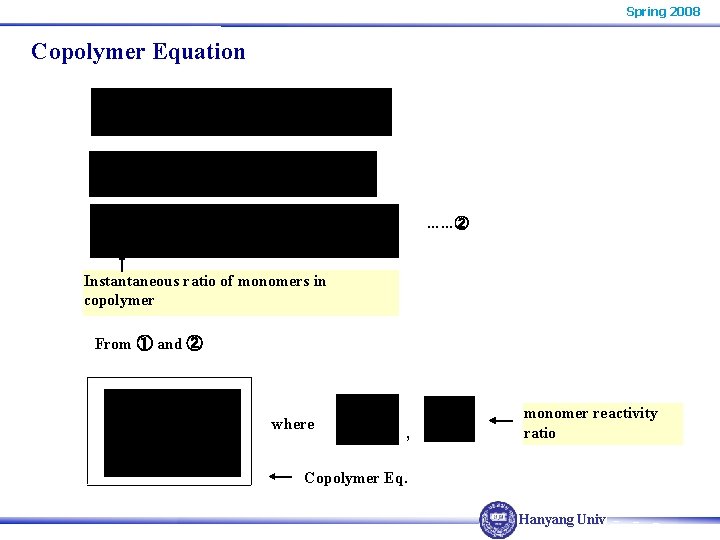
Spring 2008 Copolymer Equation ……② Instantaneous ratio of monomers in copolymer From ① and ② where , monomer reactivity ratio Copolymer Eq. Hanyang Univ.

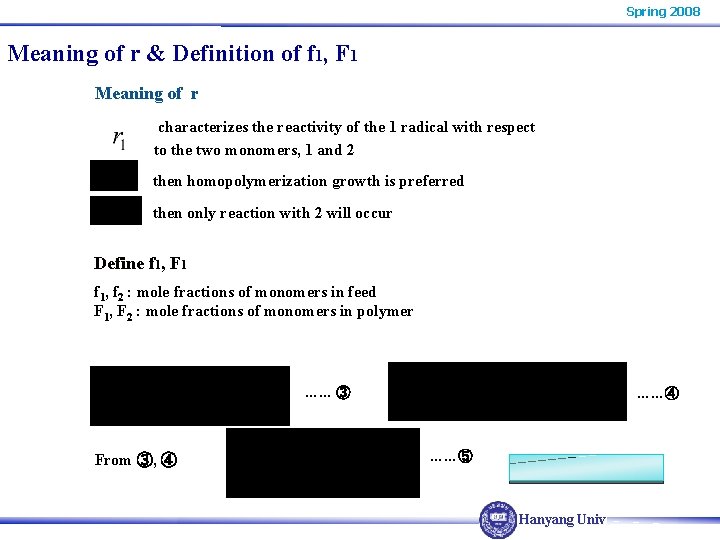
Spring 2008 Meaning of r & Definition of f 1, F 1 Meaning of r characterizes the reactivity of the 1 radical with respect to the two monomers, 1 and 2 then homopolymerization growth is preferred then only reaction with 2 will occur Define f 1, F 1 f 1, f 2 : mole fractions of monomers in feed F 1, F 2 : mole fractions of monomers in polymer …… ③ From ③, ④ ……⑤ Hanyang Univ.

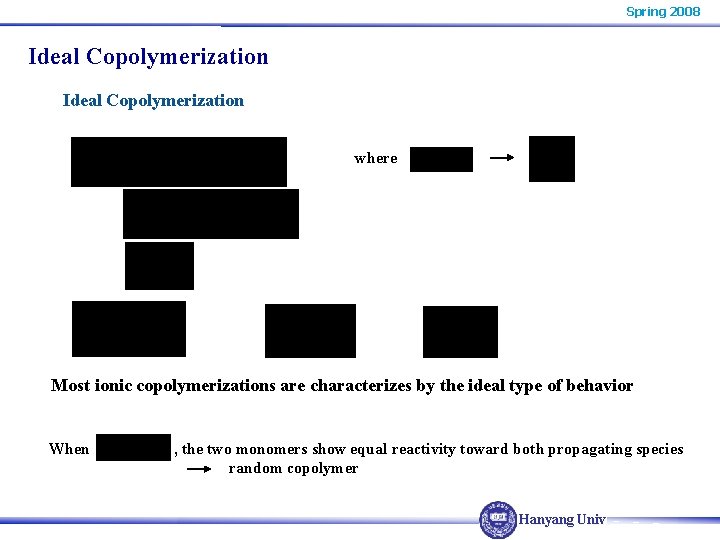
Spring 2008 Ideal Copolymerization where Most ionic copolymerizations are characterizes by the ideal type of behavior When , the two monomers show equal reactivity toward both propagating species random copolymer Hanyang Univ.

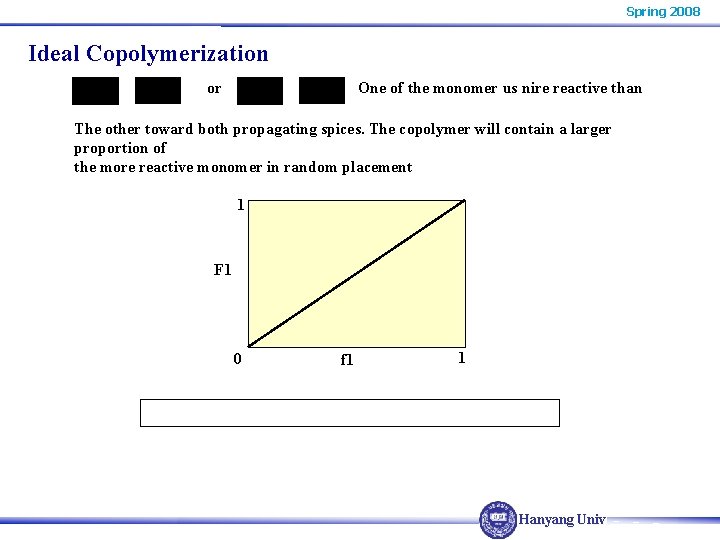
Spring 2008 Ideal Copolymerization or One of the monomer us nire reactive than The other toward both propagating spices. The copolymer will contain a larger proportion of the more reactive monomer in random placement 1 F 1 0 f 1 1 Hanyang Univ.

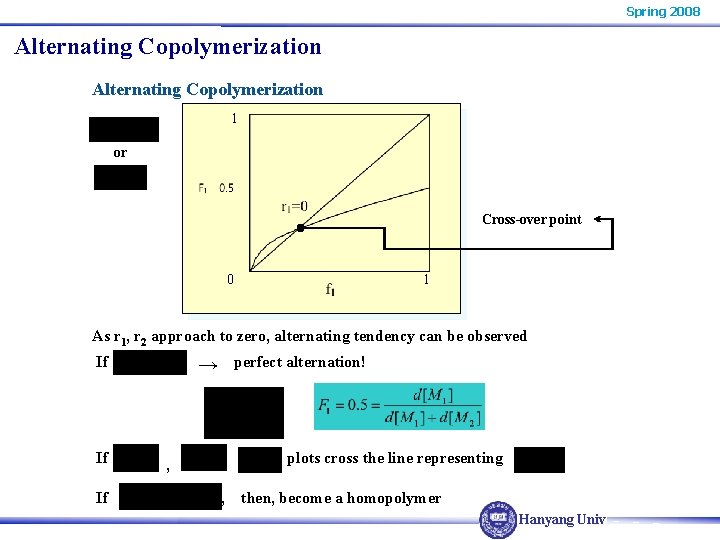
Spring 2008 Alternating Copolymerization 1 or Cross-over point 0 1 As r 1, r 2 approach to zero, alternating tendency can be observed If If If perfect alternation! → plots cross the line representing , , then, become a homopolymer Hanyang Univ.

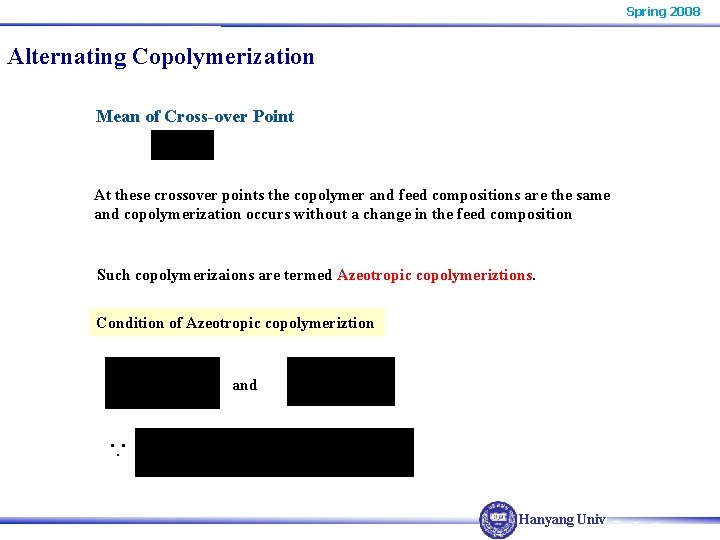
Spring 2008 Alternating Copolymerization Mean of Cross-over Point At these crossover points the copolymer and feed compositions are the same and copolymerization occurs without a change in the feed composition Such copolymerizaions are termed Azeotropic copolymeriztions Condition of Azeotropic copolymeriztion and ∵ Hanyang Univ.

Spring 2008 Alternating Copolymerization ∴ Hanyang Univ.

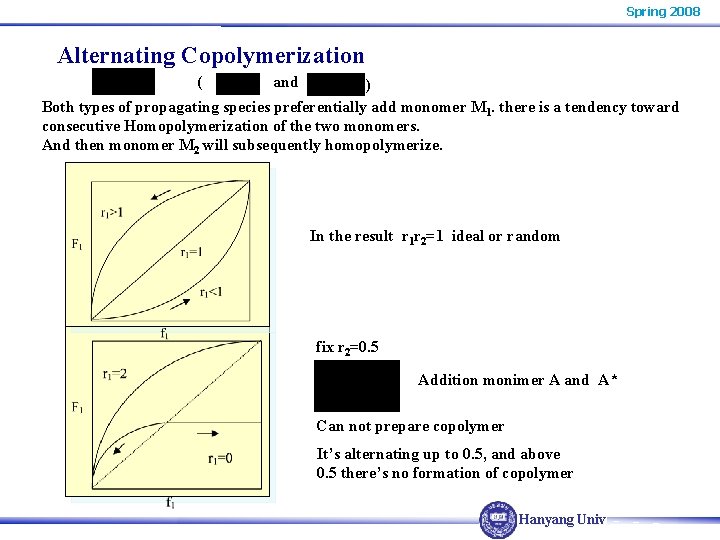
Spring 2008 Alternating Copolymerization ( and ) Both types of propagating species preferentially add monomer M 1. there is a tendency toward consecutive Homopolymerization of the two monomers. And then monomer M 2 will subsequently homopolymerize. In the result r 1 r 2=1 ideal or random fix r 2=0. 5 Addition monimer A and A* Can not prepare copolymer It’s alternating up to 0. 5, and above 0. 5 there’s no formation of copolymer Hanyang Univ.

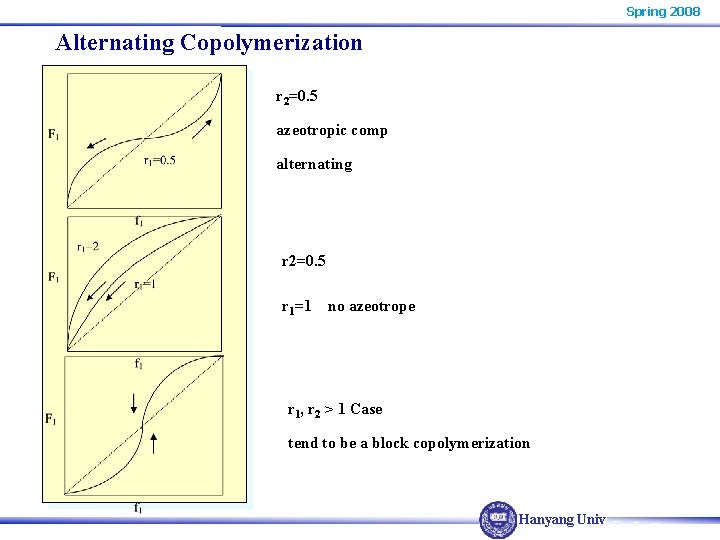
Spring 2008 Alternating Copolymerization r 2=0. 5 azeotropic comp alternating r 2=0. 5 r 1=1 no azeotrope r 1, r 2 > 1 Case tend to be a block copolymerization Hanyang Univ.

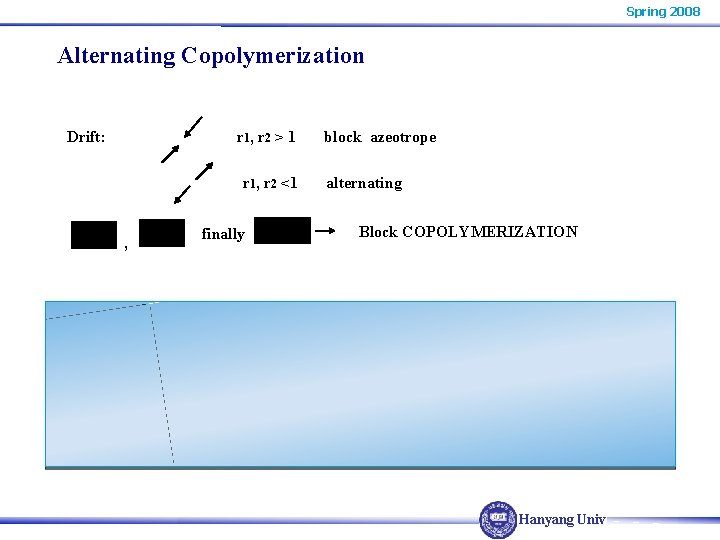
Spring 2008 Alternating Copolymerization Drift: r 1, r 2 > 1 r 1, r 2 <1 , finally block azeotrope alternating Block COPOLYMERIZATION Hanyang Univ.

Spring 2008 Experimental Determination of r 1 & r 2 1. Mayo and Lewis rearrange copolymer eq. and can get monomer comp copolymer comp. then vary r 1 value (put) and iterate Hanyang Univ.

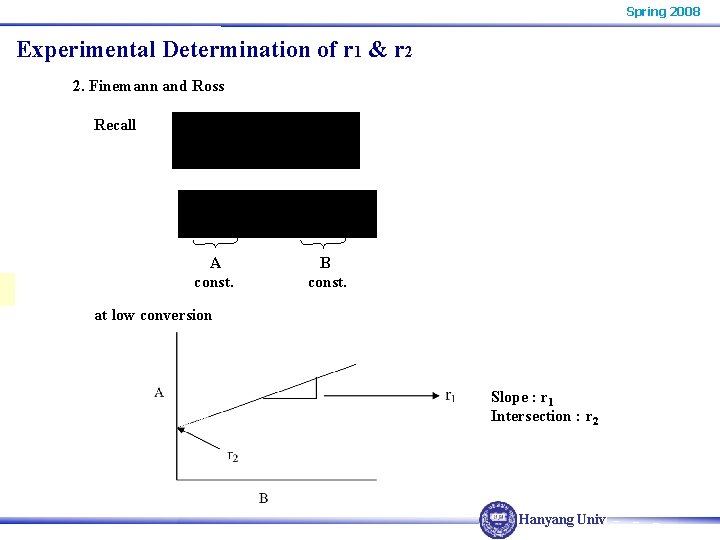
Spring 2008 Experimental Determination of r 1 & r 2 2. Finemann and Ross Recall A const. B const. at low conversion Slope : r 1 Intersection : r 2 Hanyang Univ.

Spring 2008 Relationship Between ξand F 1, f 1 Relationship Between ξ and F 1, f 1 Material Balance for M 1 where [M] = total # of moles of monomers decrease of M 1 monomer Hanyang Univ.

Spring 2008 Effect of Reaction Condition Reaction medium Depend on Solubility, PH, Viscosity, and Polarity Temperature But the effect of temperature on r is not large Pressure But the effect of pressure on r is not large Reactivity Next page Hanyang Univ.

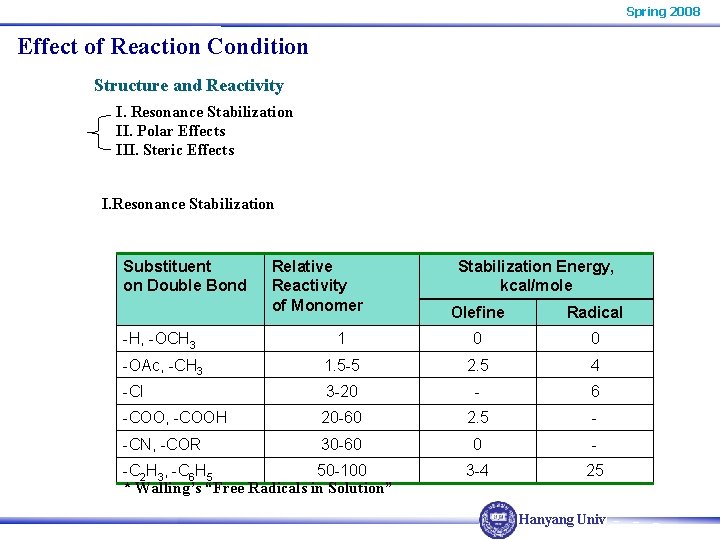
Spring 2008 Effect of Reaction Condition Structure and Reactivity I. Resonance Stabilization II. Polar Effects III. Steric Effects I. Resonance Stabilization Substituent on Double Bond Relative Reactivity of Monomer Stabilization Energy, kcal/mole Olefine Radical -H, -OCH 3 1 0 0 -OAc, -CH 3 1. 5 -5 2. 5 4 -Cl 3 -20 - 6 -COO, -COOH 20 -60 2. 5 - -CN, -COR 30 -60 0 - 3 -4 25 -C 2 H 3, -C 6 H 5 50 -100 * Walling’s “Free Radicals in Solution” Hanyang Univ.

Spring 2008 Structure and Reactivity Define r. A, r. B : monomer reactivity ratios RA, RB : active center reactivity ratios Hanyang Univ.

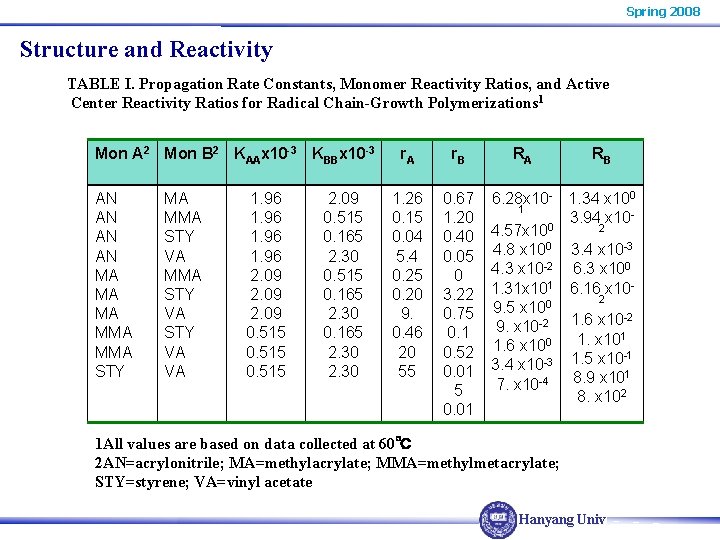
Spring 2008 Structure and Reactivity TABLE I. Propagation Rate Constants, Monomer Reactivity Ratios, and Active Center Reactivity Ratios for Radical Chain-Growth Polymerizations 1 Mon A 2 Mon B 2 KAAx 10 -3 KBBx 10 -3 AN AN MA MA MA MMA STY VA STY VA VA 1. 96 2. 09 0. 515 2. 09 0. 515 0. 165 2. 30 r. A r. B 1. 26 0. 15 0. 04 5. 4 0. 25 0. 20 9. 0. 46 20 55 0. 67 1. 20 0. 40 0. 05 0 3. 22 0. 75 0. 1 0. 52 0. 01 5 0. 01 RA RB 6. 28 x 10 - 1. 34 x 100 1 3. 94 x 100 2 4. 57 x 10 4. 8 x 100 3. 4 x 10 -3 4. 3 x 10 -2 6. 3 x 100 1. 31 x 101 6. 16 x 102 9. 5 x 100 -2 9. x 10 -2 1. 6 x 101 1. x 10 1. 6 x 100 -1 3. 4 x 10 -3 1. 5 x 10 1 8. 9 x 10 7. x 10 -4 8. x 102 1 All values are based on data collected at 60℃ 2 AN=acrylonitrile; MA=methylacrylate; MMA=methylmetacrylate; STY=styrene; VA=vinyl acetate Hanyang Univ.

Spring 2008 Structure and Reactivity Active Center Reactivity Ratios vs. Monomer Reactivity Ratios when then when The effect in relative reactivity of the active center is more stronger than that of monomer. The monomer reactivity gets affect in opposite way comparing to the active center reactivity. Hanyang Univ.

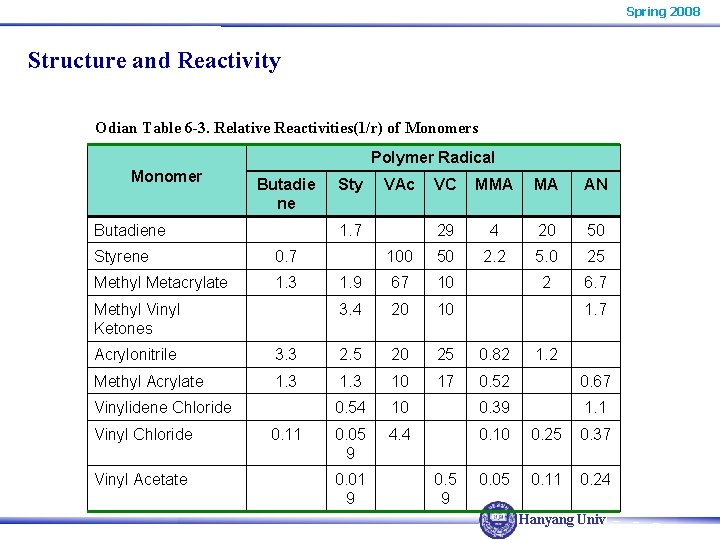
Spring 2008 Structure and Reactivity Odian Table 6 -3. Relative Reactivities(1/r) of Monomers Polymer Radical Monomer Butadie ne Butadiene Sty VAc VC MMA MA AN 29 4 20 50 100 50 2. 2 5. 0 25 1. 9 67 10 2 6. 7 3. 4 20 10 1. 7 Styrene 0. 7 Methyl Metacrylate 1. 3 Methyl Vinyl Ketones 1. 7 Acrylonitrile 3. 3 2. 5 20 25 0. 82 Methyl Acrylate 1. 3 10 17 0. 52 0. 67 0. 54 10 0. 39 1. 1 0. 05 9 4. 4 0. 10 0. 25 0. 37 0. 05 0. 11 0. 24 Vinylidene Chloride Vinyl Acetate 0. 11 0. 01 9 0. 5 9 1. 2 Hanyang Univ.

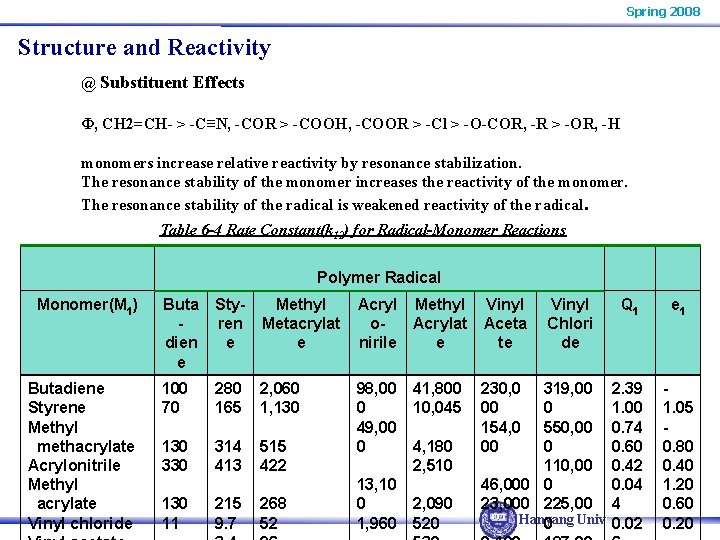
Spring 2008 Structure and Reactivity @ Substituent Effects Φ, CH 2=CH- > -C≡N, -COR > -COOH, -COOR > -Cl > -O-COR, -R > -OR, -H monomers increase relative reactivity by resonance stabilization. The resonance stability of the monomer increases the reactivity of the monomer. The resonance stability of the radical is weakened reactivity of the radical. Table 6 -4 Rate Constant(k 12) for Radical-Monomer Reactions Polymer Radical Monomer(M 1) Butadiene Styrene Methyl _methacrylate Acrylonitrile Methyl _acrylate Vinyl chloride Buta dien e Styren e Methyl Metacrylat e Acryl Methyl o. Acrylat nirile e Vinyl Aceta te 100 70 280 165 2, 060 1, 130 330 314 413 515 422 98, 00 0 49, 00 0 230, 0 00 154, 0 00 130 11 215 9. 7 268 52 13, 10 0 1, 960 41, 800 10, 045 4, 180 2, 510 2, 090 520 Vinyl Chlori de Q 1 319, 00 2. 39 0 1. 00 550, 00 0. 74 0 0. 60 110, 00 0. 42 46, 000 0 0. 04 23, 000 225, 00 4 Hanyang Univ. 0. 02 0 e 1 1. 05 0. 80 0. 40 1. 20 0. 60 0. 20

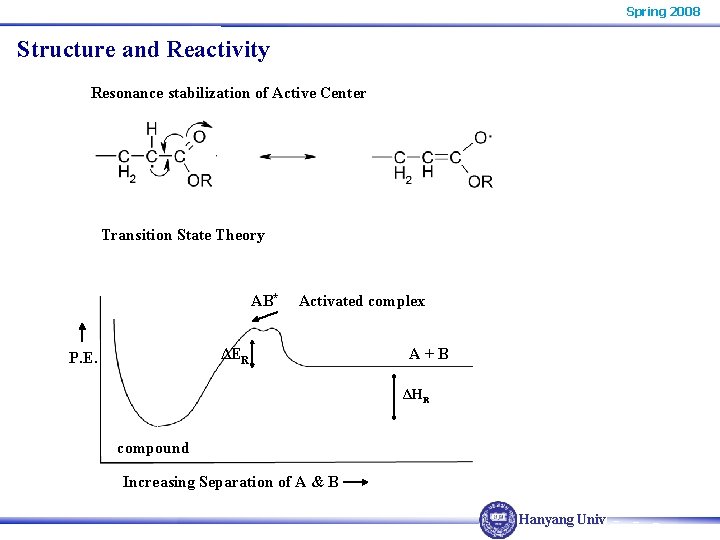
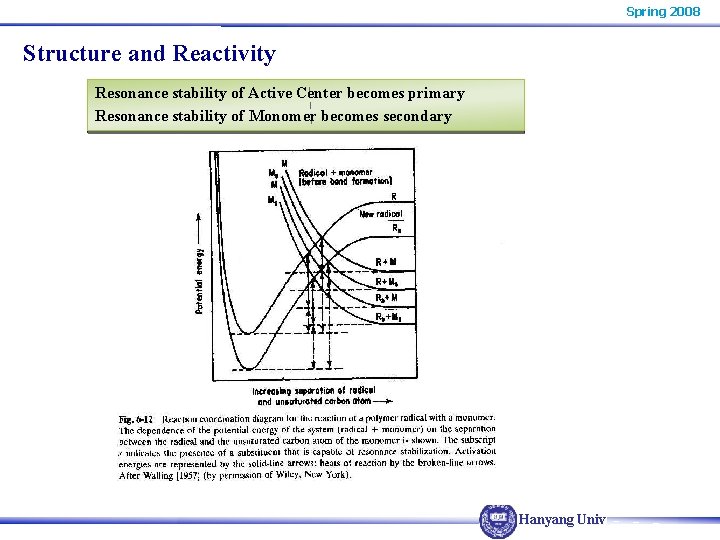
Spring 2008 Structure and Reactivity Resonance stabilization of Active Center Transition State Theory AB* Activated complex ΔER P. E. A+B ΔHR compound Increasing Separation of A & B Hanyang Univ.

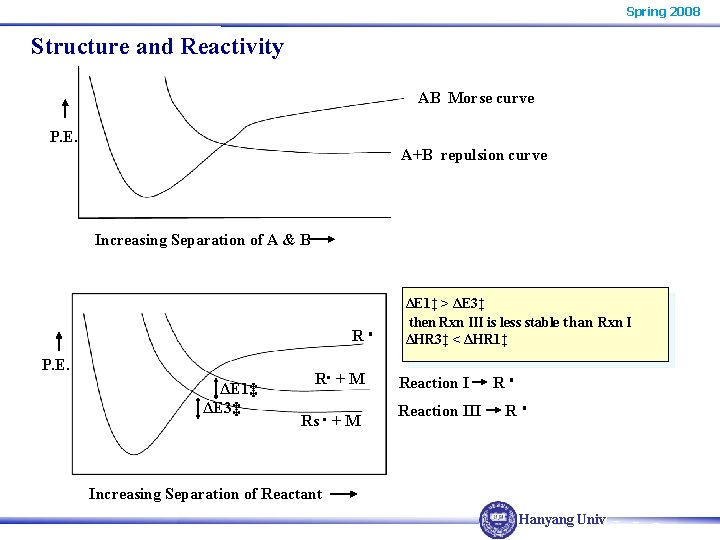
Spring 2008 Structure and Reactivity AB Morse curve P. E. A+B repulsion curve Increasing Separation of A & B R P. E. ΔE 1‡ ΔE 3‡ R + M Rs + M ΔE 1‡ > ΔE 3‡ then Rxn III is less stable than Rxn I ΔHR 3‡ < ΔHR 1‡ Reaction III R R Increasing Separation of Reactant Hanyang Univ.

Spring 2008 Structure and Reactivity Resonance stability of Active Center becomes primary Resonance stability of Monomer becomes secondary Hanyang Univ.

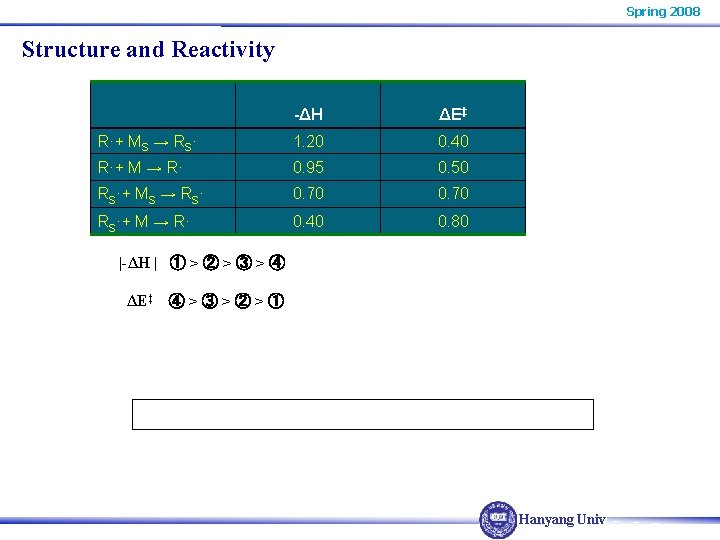
Spring 2008 Structure and Reactivity -ΔH ΔE‡ R·+ MS → RS· 1. 20 0. 40 R·+ M → R· 0. 95 0. 50 RS·+ MS → RS· 0. 70 RS·+ M → R· 0. 40 0. 80 |-ΔH | ① > ② > ③ > ④ ΔE‡ ④ > ③ > ② > ① Hanyang Univ.

Spring 2008 Structure and Reactivity II. Polar Effects Tend to cause alternation in a copolymerization i. e. for polar effects e : tendency to give monomer a polar effects Alfrey-Price Q, e scheme (Polarity Values) Hanyang Univ.

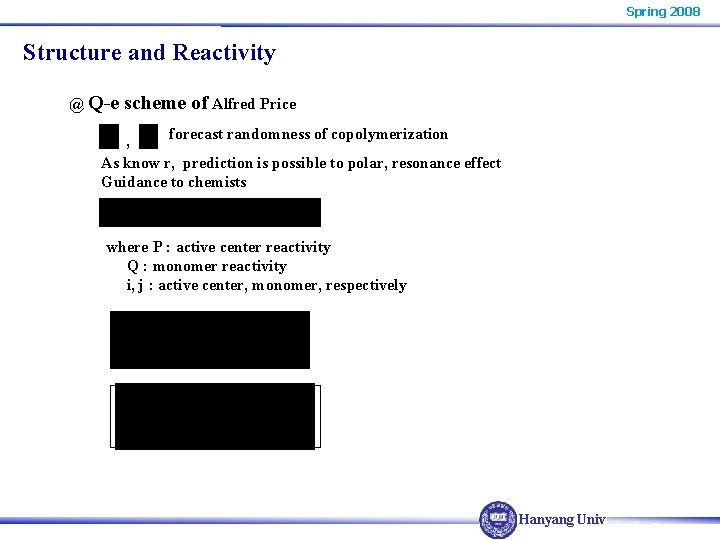
Spring 2008 Structure and Reactivity @ Q-e scheme of Alfred Price forecast randomness of copolymerization , As know r, prediction is possible to polar, resonance effect Guidance to chemists where P : active center reactivity Q : monomer reactivity i, j : active center, monomer, respectively Hanyang Univ.

Spring 2008 Structure and Reactivity So that, this equation forecasts Base materials use styrene : (arbitrary ) fair results, but not absolute in predicting r using Q-e scheme. alternating tendency is correct Hanyang Univ.

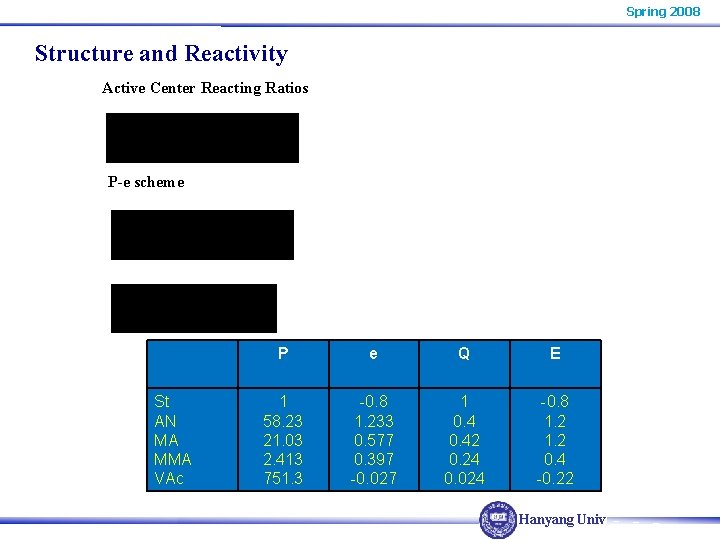
Spring 2008 Structure and Reactivity Active Center Reacting Ratios P-e scheme St AN MA MMA VAc P e Q E 1 58. 23 21. 03 2. 413 751. 3 -0. 8 1. 233 0. 577 0. 397 -0. 027 1 0. 42 0. 24 0. 024 -0. 8 1. 2 0. 4 -0. 22 Hanyang Univ.

Spring 2008 Structure and Reactivity The criticism against Q-e scheme Reference state arbitrarily set. Alternating effect was observed due to fixed charges not due to the induced dipole Exercise) How to indicate Randomness of Copolymer with Q-e scheme ? We can forecast alternation or randomness through Q-e scheme, however, why can not forecast Blockcopolymerization? (algebraic standpoint) Hanyang Univ.

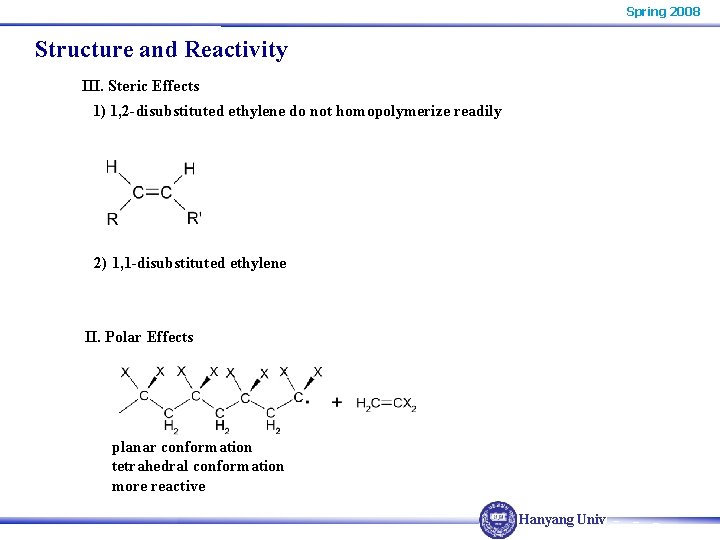
Spring 2008 Structure and Reactivity III. Steric Effects 1) 1, 2 -disubstituted ethylene do not homopolymerize readily 2) 1, 1 -disubstituted ethylene II. Polar Effects planar conformation tetrahedral conformation more reactive Hanyang Univ.

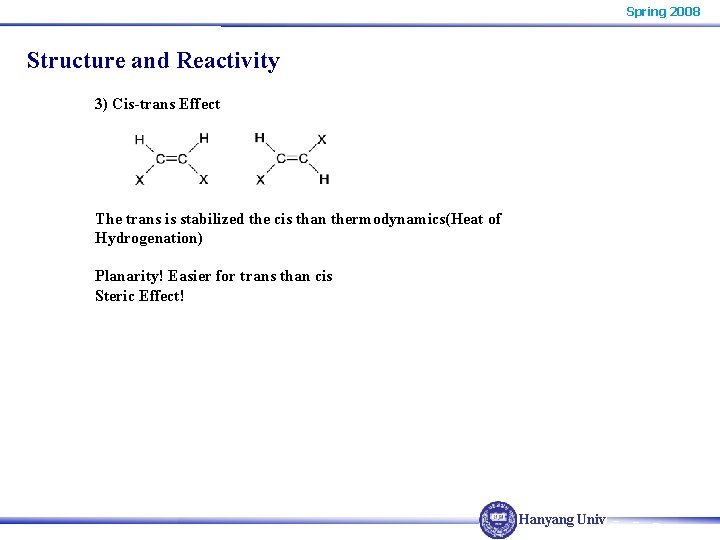
Spring 2008 Structure and Reactivity 3) Cis-trans Effect The trans is stabilized the cis than thermodynamics(Heat of Hydrogenation) Planarity! Easier for trans than cis Steric Effect! Hanyang Univ.

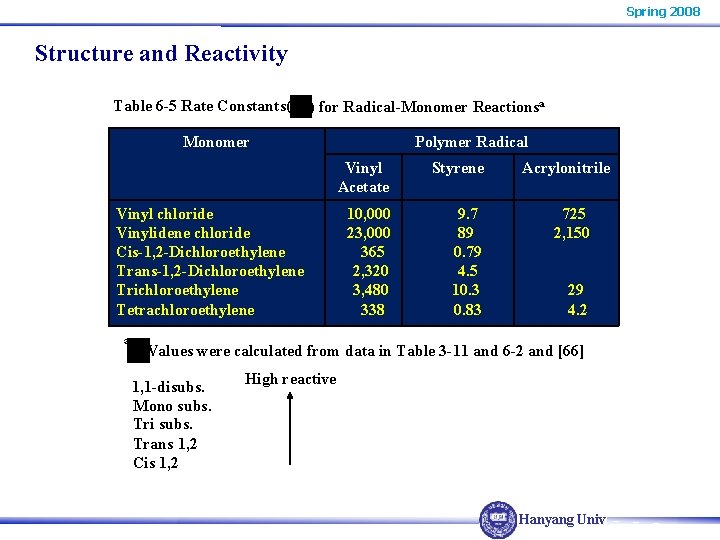
Spring 2008 Structure and Reactivity Table 6 -5 Rate Constants( ) for Radical-Monomer Reactionsa Monomer Vinyl chloride Vinylidene chloride Cis-1, 2 -Dichloroethylene Trans-1, 2 -Dichloroethylene Trichloroethylene Tetrachloroethylene a Polymer Radical Vinyl Acetate Styrene 10, 000 23, 000 365 2, 320 3, 480 338 9. 7 89 0. 79 4. 5 10. 3 0. 83 Acrylonitrile 725 2, 150 29 4. 2 Values were calculated from data in Table 3 -11 and 6 -2 and [66] 1, 1 -disubs. Mono subs. Tri subs. Trans 1, 2 Cis 1, 2 High reactive Hanyang Univ.