SpotsylvaniaStafford County Drinking Water Clinic Interpretation Meeting Virginia

































- Slides: 48

Spotsylvania/Stafford County Drinking Water Clinic Interpretation Meeting Virginia Household Water Quality Program Virginia Master Well Owner Network Virginia Cooperative Extension Biological Systems Engineering Department Virginia Tech

Why are we here? • Caring for your private water system • Well location, protection, and construction • Well maintenance and care • Drinking water regulations – knowing how much is too much • Water testing – what’s in your water? • Dealing with problems • Additional resources 2

Private Water Supplies in Virginia Majority of households in 60 of Virginia’s 95 counties rely on private water supply systems (> 1, 700, 000 people) In the U. S. municipal water supplies are regulated by the EPA under the Safe Drinking Water Act; private supplies are not! Homeowners relying on private water supplies: ◦ Are responsible for all aspects of water system management ◦ May lack knowledge and resources to effectively manage ◦ Usually don’t worry about maintenance until problems arise 3

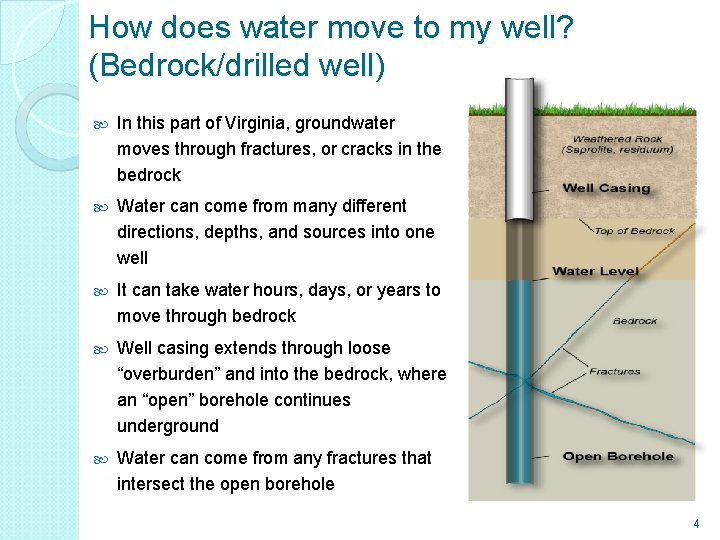
How does water move to my well? (Bedrock/drilled well) In this part of Virginia, groundwater moves through fractures, or cracks in the bedrock Water can come from many different directions, depths, and sources into one well It can take water hours, days, or years to move through bedrock Well casing extends through loose “overburden” and into the bedrock, where an “open” borehole continues underground Water can come from any fractures that intersect the open borehole 4

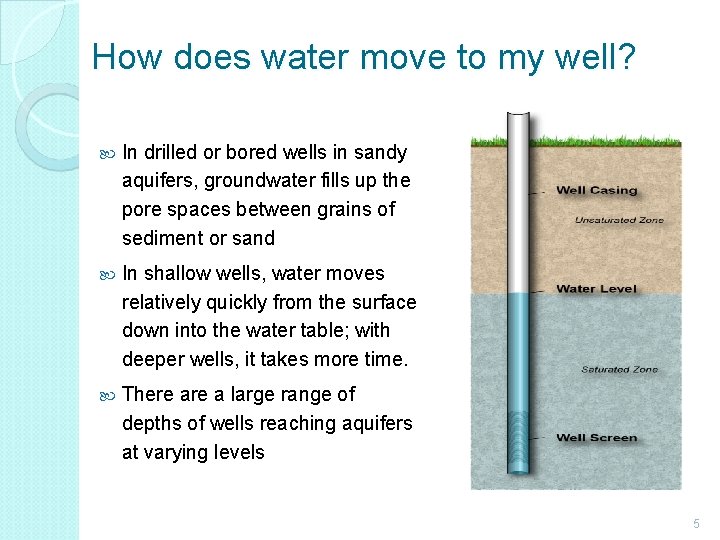
How does water move to my well? In drilled or bored wells in sandy aquifers, groundwater fills up the pore spaces between grains of sediment or sand In shallow wells, water moves relatively quickly from the surface down into the water table; with deeper wells, it takes more time. There a large range of depths of wells reaching aquifers at varying levels 5

Well Photo credit: Swistock, Penn State Univ Proper well location should be at least: ◦ 5 feet from property boundary ◦ 10 feet from building foundation (50 feet if termite treated) ◦ 50 feet from road ◦ 50 feet from sewers and septic tanks ◦ 100 feet from pastures, on-lot sewage system drainfields, cesspools or barnyards Upslope Not from potential contamination in an area that receives runoff 6

Proper well construction Contract a licensed driller: ◦ Valid Class A, B or C contractor license with WWP (Water Well and Pump) classification Well casing ◦ Minimum of 20’ for bored, 50 – 100’ deep for drilled, depending on class of well ◦ Extends 12” above ground Grouting to a minimum of 20’ Sanitary well cap or sealed concrete cover Ground slopes away from well 12” 7 Photo credits: SAIF Water Wells ; Penn State University

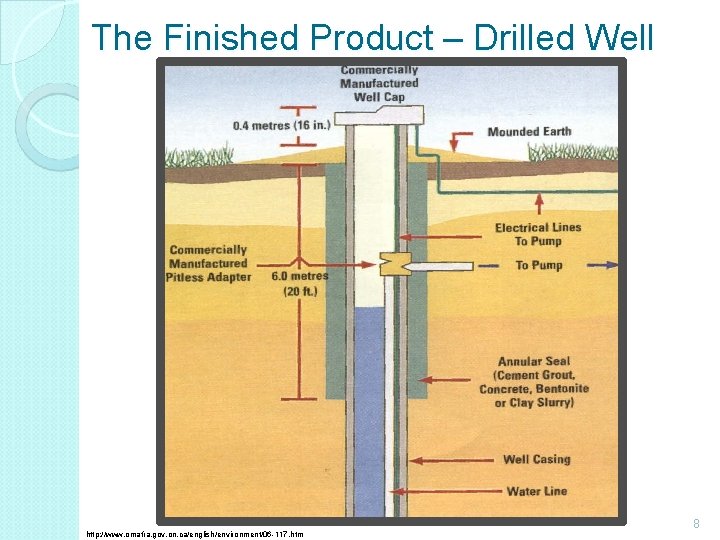
The Finished Product – Drilled Well http: //www. omafra. gov. on. ca/english/environment/06 -117. htm 8

Well Maintenance Tips Do not use fertilizers, pesticides, oil, or paint around well Keep area around well clean and accessible Keep careful records ◦ original contract, water test results and any maintenance or repair information Every year: ◦ Conduct thorough visual inspection of well ◦ Check cap for cracks, wear and tear, tightness Every 5 -10 years have well inspected by a qualified professional (with WWP classification) 9

Private Water Supply Regulations • Virginia Private Well Regulations o Specify application, inspection and construction requirements o No requirements for maintenance or water testing after construction of well – responsibility of the owner! • EPA National Drinking Water Standards o Apply to PUBLIC systems o Primary (health) and Secondary (nuisance) o Can be used as guidance for private systems to know “how much is too much” 10

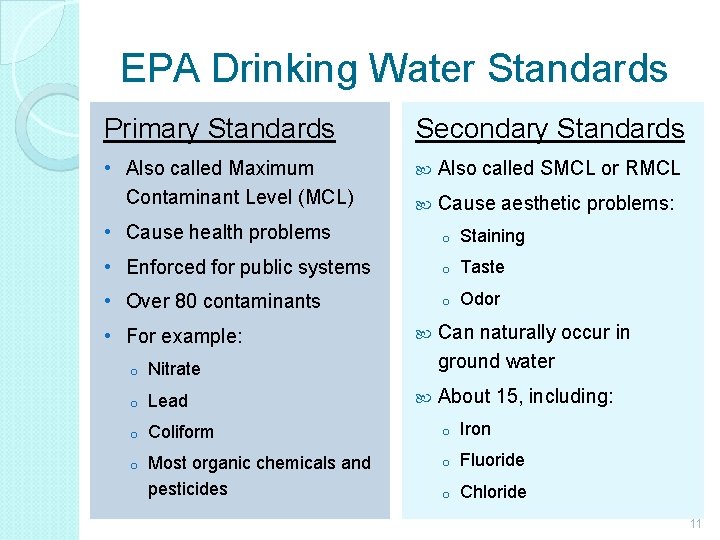
EPA Drinking Water Standards Primary Standards Secondary Standards • Also called Maximum Contaminant Level (MCL) Also called SMCL or RMCL Cause aesthetic problems: • Cause health problems o Staining • Enforced for public systems o Taste • Over 80 contaminants o Odor • For example: Can naturally occur in ground water About 15, including: o Nitrate o Lead o Coliform o Iron o Most organic chemicals and pesticides o Fluoride o Chloride 11

Testing water quality Why test? ◦ Protect family’s health and safety ◦ Many contaminants undetectable by human senses ◦ Preventive measures often more effective and less expensive ◦ Legal protection When to test? ◦ Routine tests every 1 -3 years ◦ Pregnant woman or infant in the home ◦ Recurring gastrointestinal illness ◦ Change in taste, appearance, odor of water ◦ Any services or repairs are done 12

What should I test for? Every year test for coliform bacteria ◦ Simple, relatively inexpensive test ◦ Indicates possible contamination from human or animal waste Every three years test: ◦ p. H (secondary std: 6. 5 – 8. 5) ◦ Total Dissolved Solids (TDS; secondary std 500 mg/L) ◦ Other contaminants based on local land uses nearby and condition of water 13

Conditions or nearby activities of concern Conditions or Nearby Activities Test for: Recurring gastro-intestinal illness Coliform bacteria, E. Coli Household plumbing contains metals p. H, lead, copper Corrosion of pipes and plumbing Corrosivity, p. H, lead Nearby areas of intensive agriculture Nitrate, pesticides, coliform bacteria Coal or other mining operations Metals, p. H, corrosivity Dump, junkyard or landfill VOCs, TDS, p. H, sulfate, chloride, metals Odor of gasoline or fuel oil VOCs Objectionable taste or smell of water Hydrogen sulfide, corrosivity, metals Stained plumbing fixtures or laundry Iron, copper, manganese Salty taste Chloride, TDS, sodium Scaly residues, soaps don’t lather Hardness Rapid wear of water equipment p. H, corrosivity Water is cloudy, frothy or colored Colors, detergents If you need help figuring out what to test for, call Erin! Adapted from “Drinking Water for Household Wells”, EPA, 2002 14

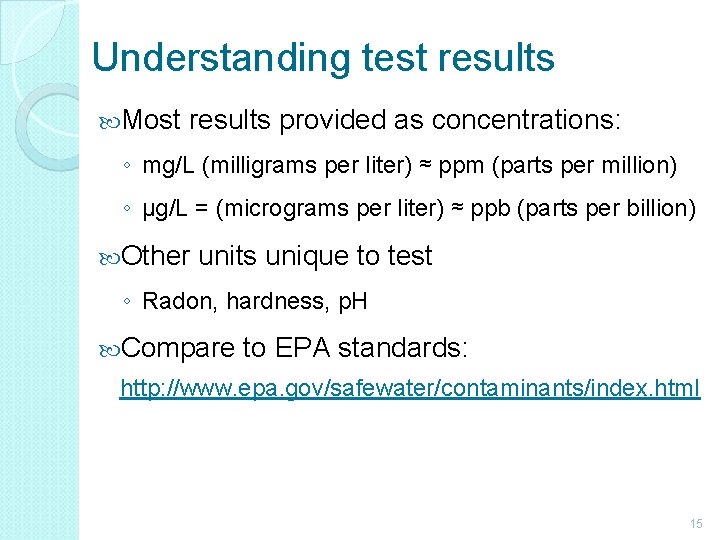
Understanding test results Most results provided as concentrations: ◦ mg/L (milligrams per liter) ≈ ppm (parts per million) ◦ µg/L = (micrograms per liter) ≈ ppb (parts per billion) Other units unique to test ◦ Radon, hardness, p. H Compare to EPA standards: http: //www. epa. gov/safewater/contaminants/index. html 15

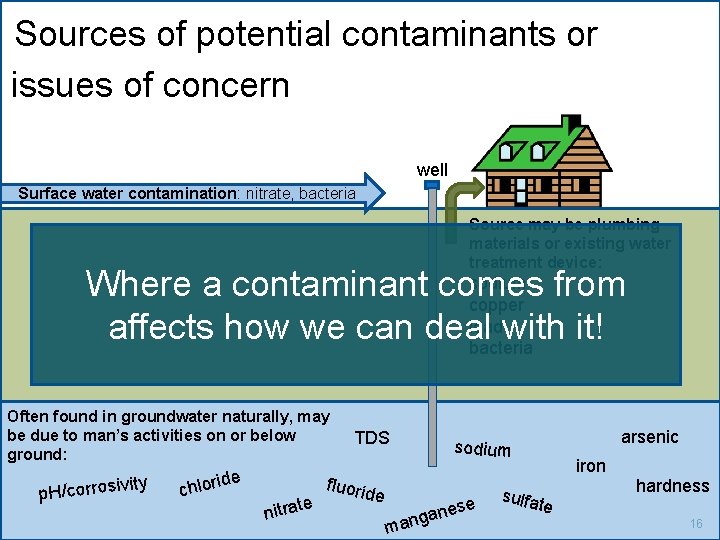
Sources of potential contaminants or issues of concern well Surface water contamination: nitrate, bacteria Source may be plumbing materials or existing water treatment device: sodium from Where a contaminant comes copper leadwith it! affects how we can deal bacteria Often found in groundwater naturally, may be due to man’s activities on or below ground: ity p. H/corrosiv c e hlorid e nitrat TDS arsenic fluorid sodium e e m nes a g n a sulfa iron te hardness 16

Options for problem water 1. If possible, control the source of pollution ◦ Divert runoff, maintain septic system 2. Improve maintenance of water system ◦ Install sanitary well cap, slope the ground 3. Treat the water to reduce contaminant concentration ◦ Match the treatment option to the pollutant ◦ Consult a professional 4. Develop a new source of water ◦ Deeper well, develop spring, connect to public water 17 http: //static. howstuffworks. com/gif/septic-tank-cleaning-1. jpg, http: //www. shipewelldrilling. com/Pictures/well_drilling_rig. jpg, http: //www. clearflow. ca/REVERSE_OSMOSIS 2. jpg

Treatment Considerations Be sure to explore ALL of your options Always have water tested by a certified lab Be aware of unscrupulous businesses – look for National Sanitation Foundation (NSF) and Water Quality Association (WQA) certifications, consult Better Business Bureau (BBB) Point of Use (POU) vs. Point of Entry (POE) Weigh benefits and limitations of a device: ◦ Cost ◦ Maintenance requirements ◦ Warranty 18

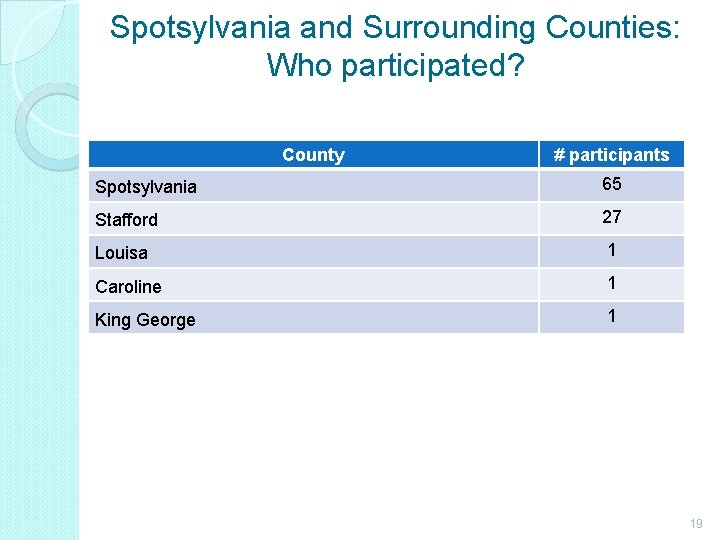
Spotsylvania and Surrounding Counties: Who participated? County # participants Spotsylvania 65 Stafford 27 Louisa 1 Caroline 1 King George 1 19

Spotsylvania and Surrounding Counties Questionnaire Results (n=95) Problems experienced with water % reporting Objectionable odor 23. 2 Staining of fixtures, appliances, laundry 53. 7 Unpleasant taste 21. 1 Corrosion of pipes and plumbing fixtures 16. 8 Unnatural color or appearance 9. 5 Presence of particles 12. 6 Sources of potential contamination % reporting Septic drain field (< 100’) 20 Heating oil storage (< 100’) 6. 3 Stream (< 100) 10. 5 Field crop operation (< 1/2 mile) 22. 1 Major farm animal operation (< 1/2 mile) 23. 2 20

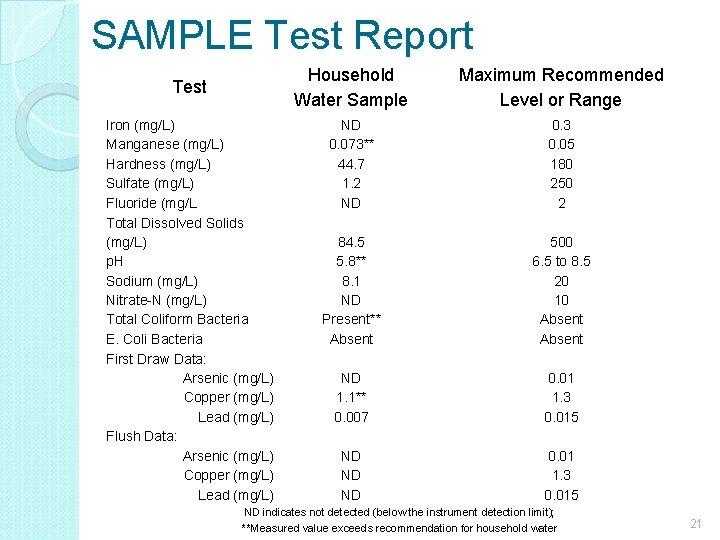
SAMPLE Test Report Test Iron (mg/L) Manganese (mg/L) Hardness (mg/L) Sulfate (mg/L) Fluoride (mg/L Total Dissolved Solids (mg/L) p. H Sodium (mg/L) Nitrate-N (mg/L) Total Coliform Bacteria E. Coli Bacteria First Draw Data: Arsenic (mg/L) Copper (mg/L) Lead (mg/L) Flush Data: Arsenic (mg/L) Copper (mg/L) Lead (mg/L) Household Water Sample Maximum Recommended Level or Range ND 0. 073** 44. 7 1. 2 ND 0. 3 0. 05 180 250 2 84. 5 5. 8** 8. 1 ND Present** Absent 500 6. 5 to 8. 5 20 10 Absent ND 1. 1** 0. 007 0. 01 1. 3 0. 015 ND ND ND 0. 01 1. 3 0. 015 ND indicates not detected (below the instrument detection limit); **Measured value exceeds recommendation for household water 21

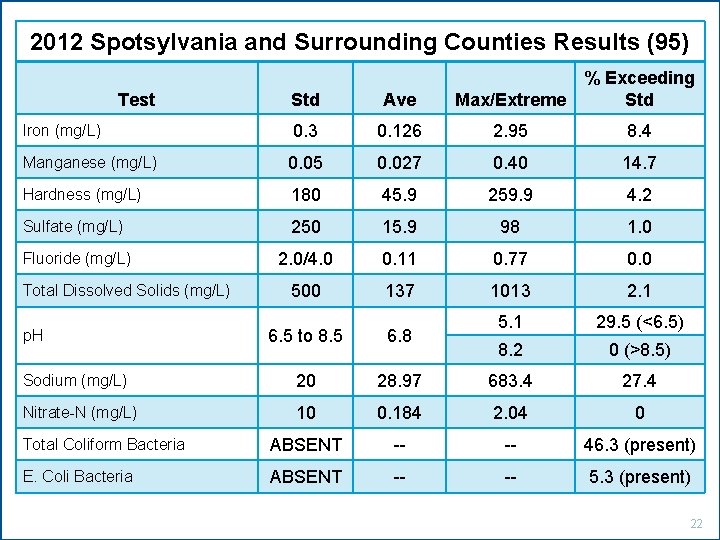
2012 Spotsylvania and Surrounding Counties Results (95) Std Ave Max/Extreme % Exceeding Std 0. 3 0. 126 2. 95 8. 4 Manganese (mg/L) 0. 05 0. 027 0. 40 14. 7 Hardness (mg/L) 180 45. 9 259. 9 4. 2 Sulfate (mg/L) 250 15. 9 98 1. 0 Fluoride (mg/L) 2. 0/4. 0 0. 11 0. 77 0. 0 500 137 1013 2. 1 5. 1 29. 5 (<6. 5) 8. 2 0 (>8. 5) Test Iron (mg/L) Total Dissolved Solids (mg/L) 6. 5 to 8. 5 6. 8 Sodium (mg/L) 20 28. 97 683. 4 27. 4 Nitrate-N (mg/L) 10 0. 184 2. 04 0 Total Coliform Bacteria ABSENT -- -- 46. 3 (present) E. Coli Bacteria ABSENT -- -- 5. 3 (present) p. H 22

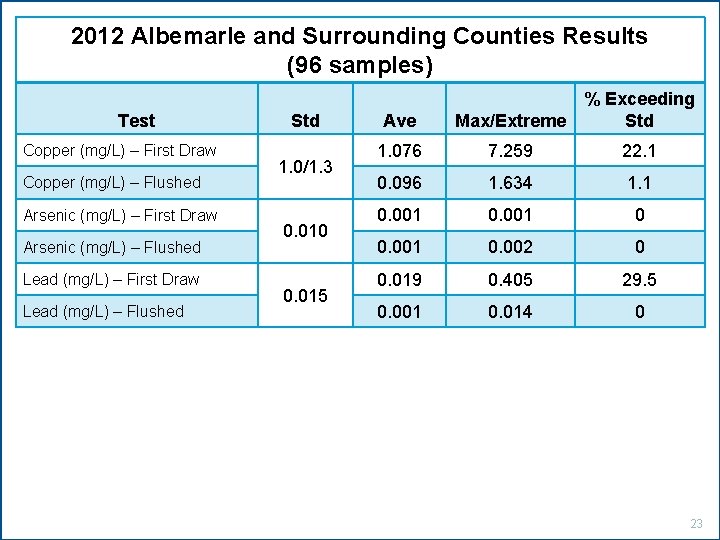
2012 Albemarle and Surrounding Counties Results (96 samples) Test Copper (mg/L) – First Draw Copper (mg/L) – Flushed Arsenic (mg/L) – First Draw Arsenic (mg/L) – Flushed Lead (mg/L) – First Draw Lead (mg/L) – Flushed Std 1. 0/1. 3 0. 010 0. 015 Ave Max/Extreme % Exceeding Std 1. 076 7. 259 22. 1 0. 096 1. 634 1. 1 0. 001 0. 002 0 0. 019 0. 405 29. 5 0. 001 0. 014 0 23

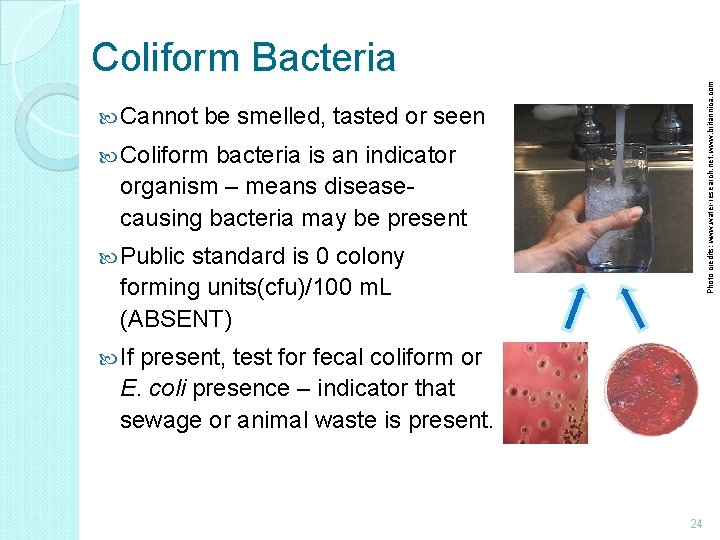
Cannot Photo credits: www. water-research. net, www. britannica. com Coliform Bacteria be smelled, tasted or seen Coliform bacteria is an indicator organism – means diseasecausing bacteria may be present Public standard is 0 colony forming units(cfu)/100 m. L (ABSENT) If present, test for fecal coliform or E. coli presence – indicator that sewage or animal waste is present. 24

If Coliform Bacteria are PRESENT Don’t panic! Examine well for pathways surface water can enter well (cracks in casing), make sure sanitary well cap is installed and secure, ground slopes away from well, etc. Consider Retest shock chlorination after shock chlorination Long term treatment options: ozonation, UV light, continuous chlorination 25

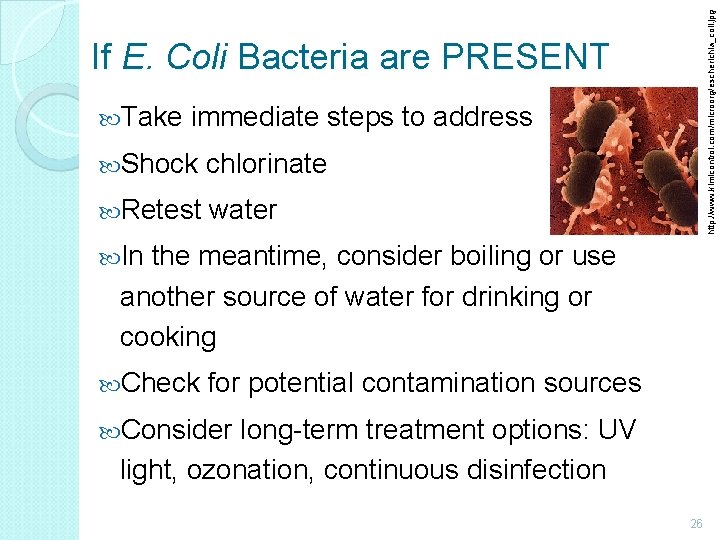
http: //www. kimicontrol. com/microorg/escherichia_coli. jpg If E. Coli Bacteria are PRESENT Take immediate steps to address Shock chlorinate Retest water In the meantime, consider boiling or use another source of water for drinking or cooking Check for potential contamination sources Consider long-term treatment options: UV light, ozonation, continuous disinfection 26

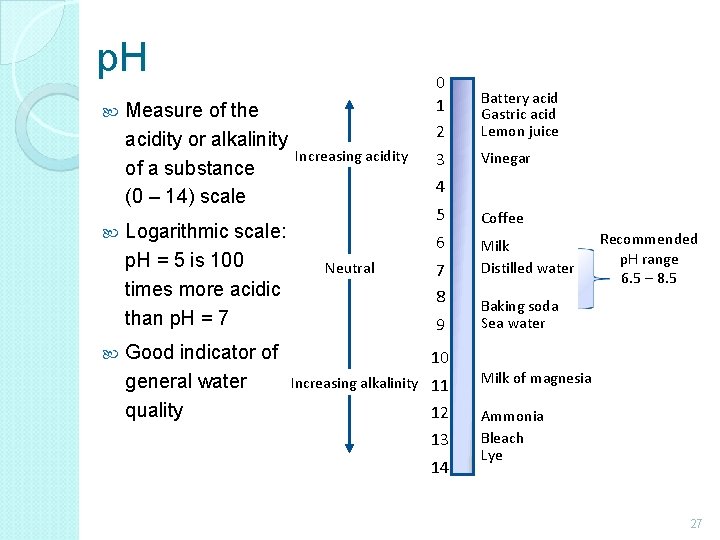
p. H Measure of the acidity or alkalinity of a substance (0 – 14) scale Logarithmic scale: p. H = 5 is 100 times more acidic than p. H = 7 Good indicator of general water quality Increasing acidity 0 1 2 Battery acid Gastric acid Lemon juice 3 Vinegar 4 Neutral 5 Coffee 6 Milk Distilled water 7 8 9 10 Increasing alkalinity 11 12 13 14 Recommended p. H range 6. 5 – 8. 5 Baking soda Sea water Milk of magnesia Ammonia Bleach Lye 27

Corrosive Water Also called aggressive water Corrodes metal plumbing – can leach metals, causes pitting and leaks, reduces length of appliance life Most commonly caused by low p. H; other contributing factors include alkalinity, temperature, TDS levels EPA recommends drinking water be non-corrosive Excess copper or lead in drinking water is a health concern Depending on p. H, treat with acid neutralizing filter or soda ash injection http: //www. bushman. cc/photos/Copper_Water_Pipe_Corrosion. jpg; http: //www. cee. vt. edu/ewr/environmental/teach/wtprimer/corrosion. html 28

Corrosive Water: Metals of concern Lead ◦ Many serious health effects, especially in children and infants Developmental, neurological, reproductive and renal ◦ EPA MCL is 0 mg/L with a health action level of 0. 015 mg/L. ◦ Sources include: Pipes in older homes (pre-1930) Solder in homes built prior to 1986 “Lead-free” brass fixtures (<8%) – even in NEW homes! Copper ◦ High levels can cause nausea, vomiting, stomach cramps; infants and children particularly sensitive ◦ EPA MCL is 1. 3 mg/L ◦ Nuisance effects noticeable at 1. 0 mg/L http: //www. gravitaexim. com/images/Lead-pipe. jpg 29

Addressing Lead or Copper in Water Options to consider: ◦ Discuss test results with your physician if concerned! ◦ Metals will be highest with corrosive water and contact time with pipes. Flushing pipes may address problem. Make sure that water runs until it is as cold as it gets before drinking ◦ Activated carbon filter (e. g. Brita) MAKE SURE IT IS LABELED TO REMOVE LEAD MAKE SURE TO CHANGE AS DIRECTED ◦ Address corrosivity of water – if p. H < 6. 5, can use acid neutralizing filter; however, corrosivity can be caused by other factors as well ◦ Reverse Osmosis ◦ Use another source of water known to be safe 30

http: //wi. water. usgs. gov/pubs/FS-221 -95/p 2. gif Nitrate (NO 3 -N) Serious health concern for infants ◦ Methemoglobinemia or “blue baby syndrome” Nitrate nitrite during digestion and blood cannot carry oxygen ◦ MCL 10 mg/L NO 3 -N or 45 mg/L of NO 3 If 3 -5 mg/L, use do not use water for infants under 6 months Sources include fertilizer, animal manure, sewage NO 3 dissolves and moves easily through soil Test in spring months; levels change over time BOILING INCREASES concentration of nitrates!!! Treatment: distillation, reverse osmosis, ion exchange 31

/www. cotrip. org/winterdriving/images/pic 6. jpg; /www. apswater. com/images/fleck%205600. jpg Sodium Low levels occur naturally; high levels may be from man-made source ◦ Road salt storage or application ◦ Industrial waste ◦ Sewage, fertilizers or animal waste ◦ WATER SOFTENER Sodium: EPA MCL for people on low-sodium diets: 20 mg/L Consider other sources of salt in diet and discuss with Dr. Higher levels may indicate contamination – test for bacteria or other contaminants Salty taste; and may accelerate corrosion of pipes and water heaters Treat using distillation, reverse osmosis, demineralization 32

Total Dissolved Solids (TDS) Water is a great solvent – dissolves many compounds as it travels over and under ground TDS is a measure of all dissolved impurities Natural sources: limestone, salt deposits, other minerals Man-made sources: ◦ Septic systems and sewage ◦ Run off from agricultural or urban land ◦ Road salt, industrial sources General indicator of water quality; test at least every three years EPA SMCL is 500 mg/L Treat using distillation or reverse osmosis http: //en. wikipedia. org/wiki/Total_dissolved_solids 33

http: //www. freedrinkingwater. com/images-water-quality/chemicals/water%20 in%20 reddish-brown. jpg Iron and Manganese Nuisance - not health concern SMCL: Iron = 0. 3 mg/L Manganese = 0. 05 mg/L Red-brown/black staining, particles, metallic taste Treatment depends on type/form of iron ◦ Ferrous: water initially clear orange-brown or black solid particles ◦ Ferric: solid particles apparent immediately, or water has a tint ◦ Iron bacteria: not a health concern; feed on Fe and Mn, forming red-brown or black-brown slime Treatment: water softener, aeration and filtration, ozonation, distillation 34

Nuisance problems http: //www. process-controls. com/techsales/Dynamic_Descaler/images/before_1. jpg, www. tamhil. com/english/content. asp? id=24 35

Nuisance problems Photo credits: www. ehrenner. com/Chloronation. html, www. bookofjoe. com/2006/01/13/index. html, cleanwellwater. com/acidic_water_bluegreen_stains 36

Nuisance problems Photo credits: Midland Corrosion Associates, www. awqinc. com/ph. html, www. ehrenner. com/Chloronation. html, http: //www. copper. org/applications/plumbing/techcorner/images/erosion_corrosion. jpg 37

What do you recall about…. . Iron and manganese Bacteria Hardness Corrosive and Scaling Water Metals Nitrate Sodium TDS Fluoride 38

Virginia Master Well Owner Network Training Workshop ore m rn your a Le out r! ab ate w New opportunity for private water supply users! Help Other s! Visit www. wellwater. bse. vt. edu today to find out more and complete an application or contact Erin Ling Free e urc o s e R er! Bind wellwater@vt. edu y Appl y! toda 540 -231 -9058 39

Erin Ling (wellwater@vt. edu) Virginia Household Water Quality Program Virginia Master Well Owner Network Brian Benham (benham@vt. edu) www. wellwater. bse. vt. edu Email: wellwater@vt. edu Ph: 540 -231 -9058 40

Resources Virginia Household Water Quality Program www. wellwater. bse. vt. edu Virginia Certified Lab Listing: http: //www. wellwater. bse. vt. edu/files/lablist 2010. pdf EPA Private Wells Site http: //www. epa. gov/ogwdw/privatewells/whatyoucando. html National Groundwater Association Well Owner http: //www. wellowner. org/ Water Systems Council Wellcare Hotline http: //www. wellcarehotline. org/ National Sanitation Foundation: www. nsf. org Water Quality Association: www. wqa. org Consumer Reports or Better Business Bureau www. consumerreports. org OR www. bbb. org 41

End of slide show. Additional slides follow if you wish to add them. 42

www. goodcleanwater. com/fyi. htm; www. watersoftening. org/effects_of_hard_water. htm; Hardness/Scaling Hard water contains high levels of calcium and magnesium ions ◦ Dissolved into water during contact with limestone and other minerals Not a health risk – nuisance ◦ Decreased cleaning action of soaps, detergents ◦ Scale build-up in pipes and on appliances ◦ Reduced efficiency and lifespan of water heaters No EPA standard for public systems Treat using water softener Hardness Rating Grains per Gallon mg/L Soft Less than 1. 0 Less than 17. 1 Slightly-Moderately Hard 1. 0 -7. 0 17. 1 -120 Hard 7. 0 -10. 5 120 -180 Very Hard Over 10. 5 Over 180 43

Fluoride Occurs naturally in varying levels ◦ Naturally high levels of F in E. Virginia groundwater Added to many public water systems for reduced dental caries and strong teeth and bones Health concerns: ◦ Long term exposure: links to bone cancer ◦ Shorter term exposure: dental or skeletal fluorosis EPA MCL 4. 0 mg/L and SMCL 2. 0 mg/L Optimum Limited levels for public systems 0. 8 - 1. 2 mg/L use for children up to 8 years Treatment (reverse osmosis) removes ALL fluoride http: //www. willamettedental. com/en_us/ALL/patients/pps/retailproducts_prettysmile. gif; http: //en. wikipedia. org/wiki/Dental_fluorosis 44

Arsenic Occurs naturally in some rocks; more common in groundwater supplies when water tables rise and fall frequently Used in wood preservatives, paints, pesticides, etc. Linked to many types of cancer, stomach pain, paralysis, and blindness EPA primary standard is 0. 010 mg/L Reverse osmosis to remove 45

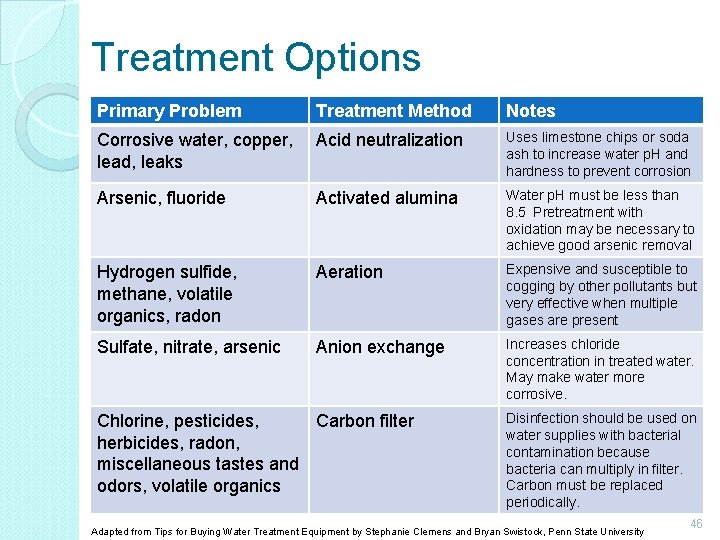
Treatment Options Primary Problem Treatment Method Notes Corrosive water, copper, lead, leaks Acid neutralization Uses limestone chips or soda ash to increase water p. H and hardness to prevent corrosion Arsenic, fluoride Activated alumina Water p. H must be less than 8. 5 Pretreatment with oxidation may be necessary to achieve good arsenic removal Hydrogen sulfide, methane, volatile organics, radon Aeration Expensive and susceptible to cogging by other pollutants but very effective when multiple gases are present Sulfate, nitrate, arsenic Anion exchange Increases chloride concentration in treated water. May make water more corrosive. Chlorine, pesticides, Carbon filter herbicides, radon, miscellaneous tastes and odors, volatile organics Disinfection should be used on water supplies with bacterial contamination because bacteria can multiply in filter. Carbon must be replaced periodically. Adapted from Tips for Buying Water Treatment Equipment by Stephanie Clemens and Bryan Swistock, Penn State University 46

Treatment Options Primary Problem Treatment Method Notes Bacteria, iron and manganese Chlorination Water must be clear for chlorine to work. Requires tank for storage and contact time. Removes everything except volatile organics, pesticides, herbicides Distillation Produces small amounts of bland -tasting water. Space needed to store treated water. Iron, manganese, hydrogen sulfide Oxidizing filters Periodic addition of chemicals and backwashing is necessary. Good option when all three are present. Bacteria, metals, odors, tastes Ozone Expensive to purchase and operate but very effective at removing multiple pollutants. All dissolved pollutants Reverse osmosis Produces small amounts of water and some waste water. Will not remove most organic pollutants or bacteria Adapted from Tips for Buying Water Treatment Equipment by Stephanie Clemens and Bryan Swistock, Penn State University 47

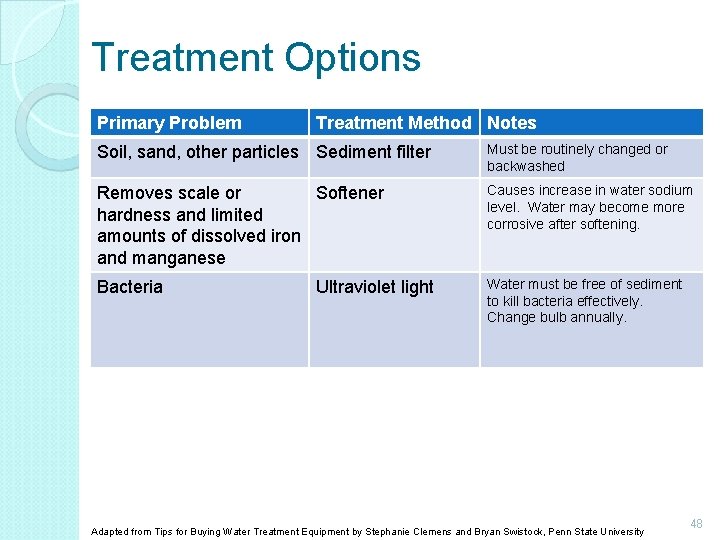
Treatment Options Primary Problem Treatment Method Notes Soil, sand, other particles Sediment filter Must be routinely changed or backwashed Removes scale or Softener hardness and limited amounts of dissolved iron and manganese Causes increase in water sodium level. Water may become more corrosive after softening. Bacteria Water must be free of sediment to kill bacteria effectively. Change bulb annually. Ultraviolet light Adapted from Tips for Buying Water Treatment Equipment by Stephanie Clemens and Bryan Swistock, Penn State University 48
 Water and water and water water
Water and water and water water Excessive drinking by county
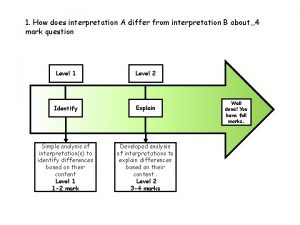
Excessive drinking by county How does interpretation b differ from interpretation a
How does interpretation b differ from interpretation a Drinking water watch texas
Drinking water watch texas Drinking water state revolving fund
Drinking water state revolving fund Oregon wastewater operator certification
Oregon wastewater operator certification Drinking water watch indiana
Drinking water watch indiana Texas water watch
Texas water watch Lithium in drinking water map
Lithium in drinking water map Typhoid medicine course
Typhoid medicine course Drinking water system operator certificate
Drinking water system operator certificate Prime drinking water
Prime drinking water Nm drinking water watch
Nm drinking water watch Northern virginia seo audit
Northern virginia seo audit Travis county wellness clinic
Travis county wellness clinic Sleep study near grayson county nc
Sleep study near grayson county nc Today meeting or today's meeting
Today meeting or today's meeting Proposal kickoff meeting agenda
Proposal kickoff meeting agenda What is meeting and types of meeting
What is meeting and types of meeting What is meeting and types of meeting
What is meeting and types of meeting Southampton county, virginia plantations
Southampton county, virginia plantations Middlesex county virginia
Middlesex county virginia Hamblen county school board meeting
Hamblen county school board meeting Tccg ground
Tccg ground Joint meeting essex and union county
Joint meeting essex and union county Important water features of virginia
Important water features of virginia Virginia water features
Virginia water features Somerset mold removal
Somerset mold removal Water treatment rockingham county
Water treatment rockingham county Volusia county water and utilities
Volusia county water and utilities Vance county water district
Vance county water district Genesee waste
Genesee waste Volusia county water resources and utilities
Volusia county water resources and utilities Water mitigation pike county
Water mitigation pike county King henry once upon a time
King henry once upon a time Teenage alcohol consumption
Teenage alcohol consumption Metric system
Metric system Youtube youtube
Youtube youtube Deka drank
Deka drank Once upon a time in a far away land
Once upon a time in a far away land King henry died drinking chocolate milk metric system
King henry died drinking chocolate milk metric system Km hm dcm
Km hm dcm Follow the drinking gourd lyrics
Follow the drinking gourd lyrics Drinking black coffee drivers ed
Drinking black coffee drivers ed Bmw drinking and driving ad
Bmw drinking and driving ad Kangaroos+hopping+down+stairs+drinking+chocolate+milk
Kangaroos+hopping+down+stairs+drinking+chocolate+milk Drunk jeopardy questions
Drunk jeopardy questions Who wrote follow the drinking gourd
Who wrote follow the drinking gourd Eating or drinking verbs that express anger
Eating or drinking verbs that express anger