SPONCH What is SPONCH SPONCH 6 most important

- Slides: 15

SPONCH What is SPONCH?

SPONCH 6 most important elements to life • • • S= Sulfur P= Phosphorus O= Oxygen N= Nitrogen C= Carbon H= Hydrogen

Matter • Anything that occupies space and has mass

Element • Simplest form of matter, cannot be broken down chemically into a simpler kind of matter

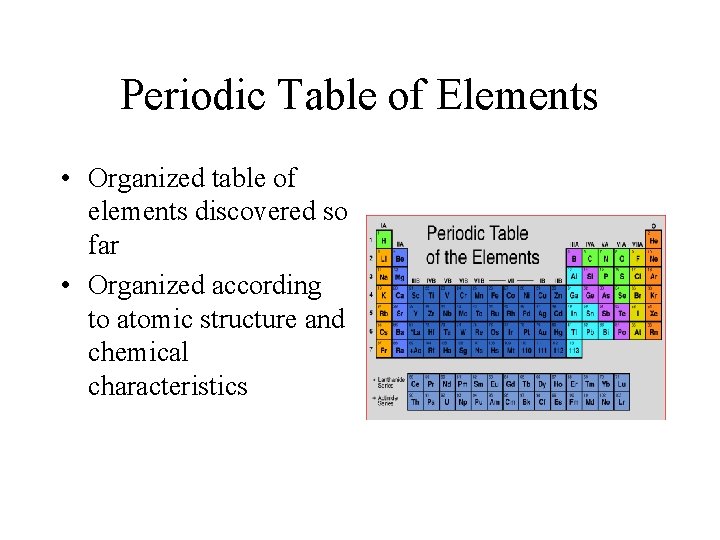
Periodic Table of Elements • Organized table of elements discovered so far • Organized according to atomic structure and chemical characteristics

Atoms and Atomic Structure • Atoms are the simplest form of an element that keeps all the properties of the element

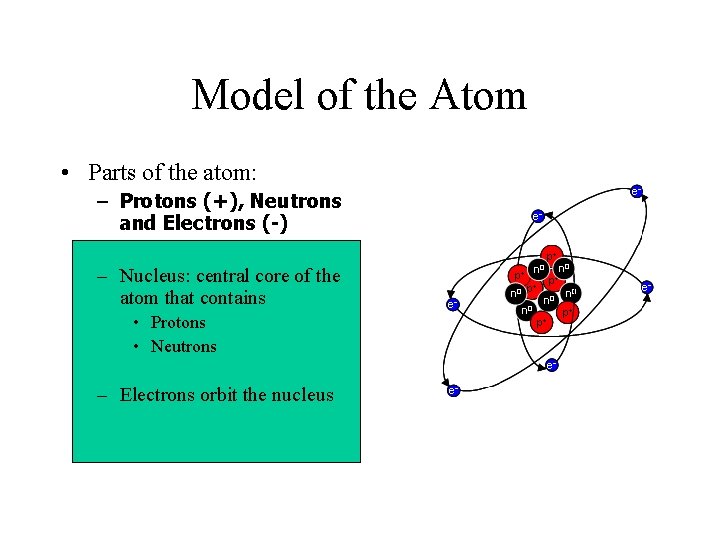
Model of the Atom • Parts of the atom: – Protons (+), Neutrons and Electrons (-) – Nucleus: central core of the atom that contains • Protons • Neutrons – Electrons orbit the nucleus

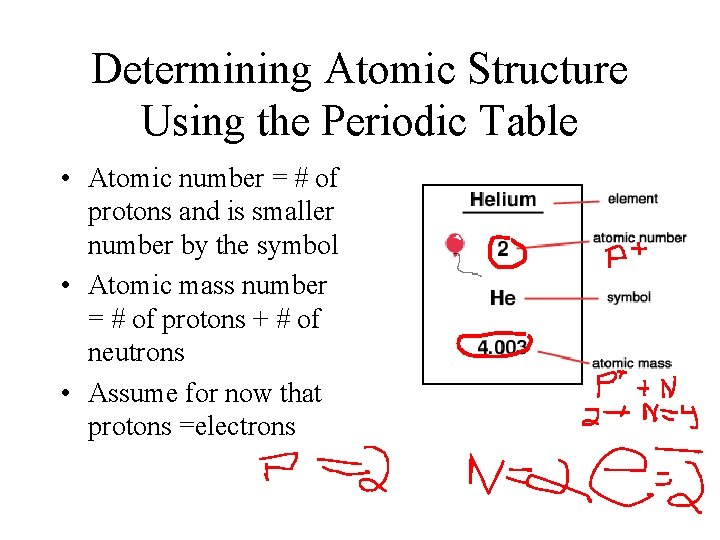
Determining Atomic Structure Using the Periodic Table • Atomic number = # of protons and is smaller number by the symbol • Atomic mass number = # of protons + # of neutrons • Assume for now that protons =electrons

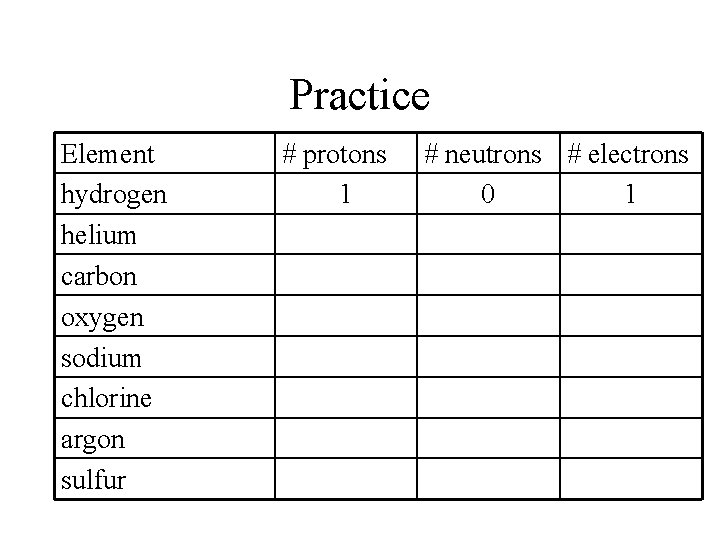
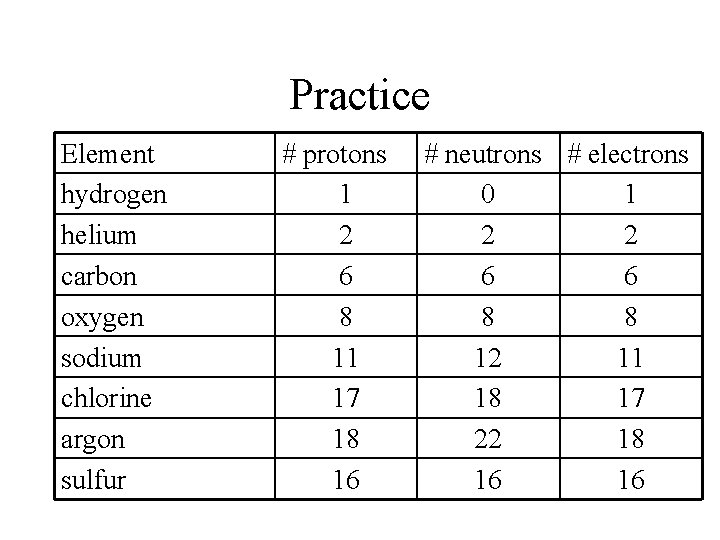
Practice Element hydrogen helium carbon oxygen sodium chlorine argon sulfur # protons 1 # neutrons # electrons 0 1

Practice Element hydrogen helium carbon oxygen sodium chlorine argon sulfur # protons 1 2 6 8 11 17 18 16 # neutrons # electrons 0 1 2 2 6 6 8 8 12 11 18 17 22 18 16 16

Types of Bonds • COVALENT – strong bond between elements • IONIC- attraction between elements due to opposite charges (weaker than covalent) • HYDROGEN – weakest type of bond

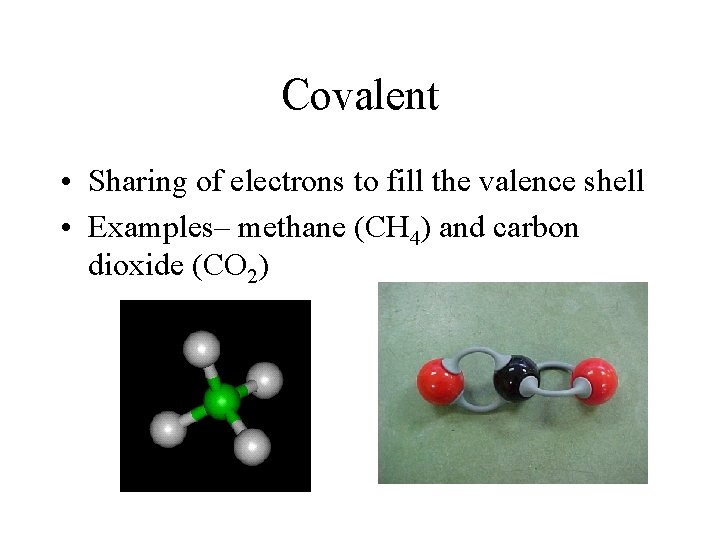
Covalent • Sharing of electrons to fill the valence shell • Examples– methane (CH 4) and carbon dioxide (CO 2)

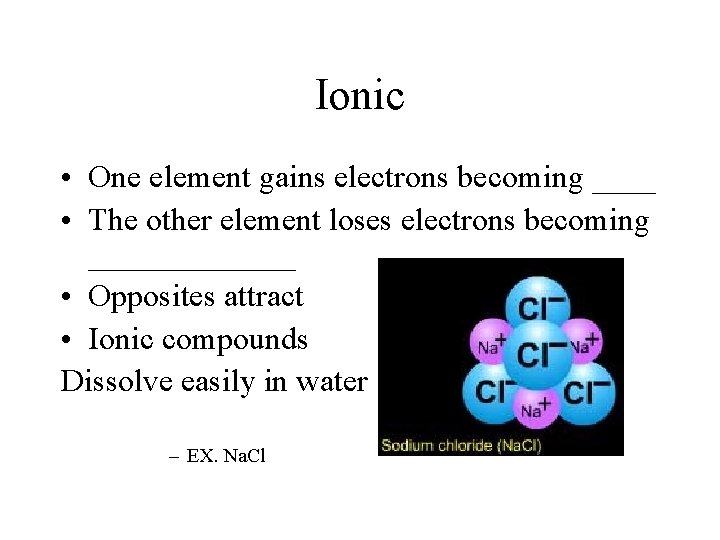
Ionic • One element gains electrons becoming ____ • The other element loses electrons becoming _______ • Opposites attract • Ionic compounds Dissolve easily in water – EX. Na. Cl

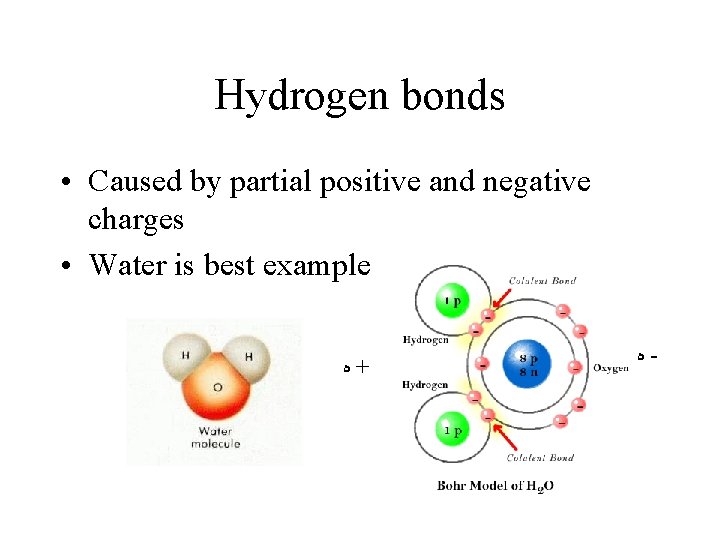
Hydrogen bonds • Caused by partial positive and negative charges • Water is best example ﮦ + ﮦ -

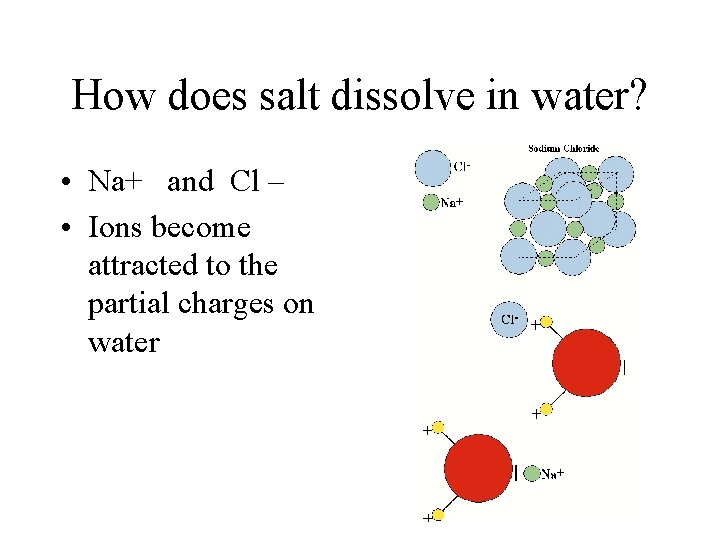
How does salt dissolve in water? • Na+ and Cl – • Ions become attracted to the partial charges on water