Spectroscopy The spectral colors correspond to different wavelengths







- Slides: 25

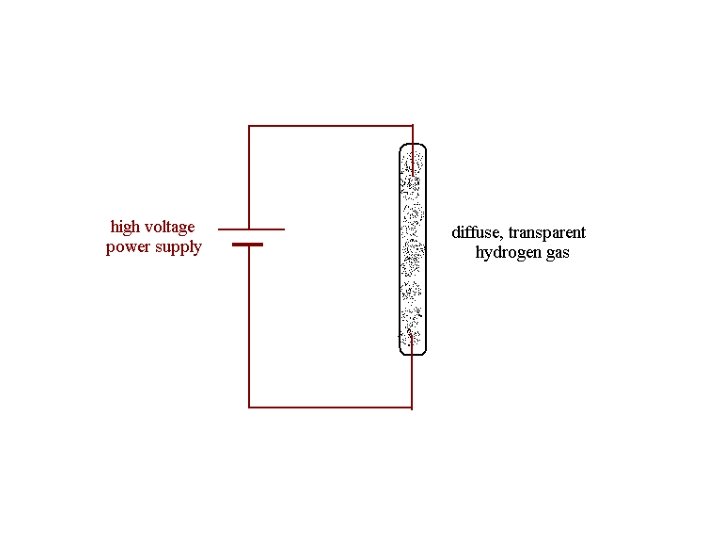
Spectroscopy

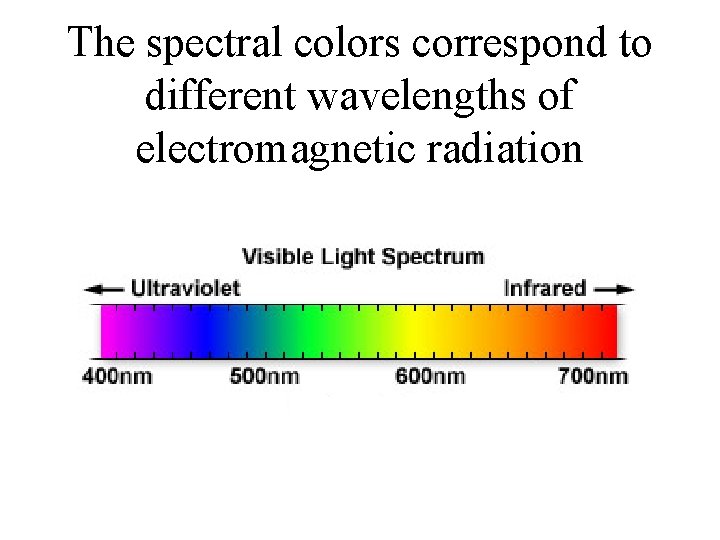
The spectral colors correspond to different wavelengths of electromagnetic radiation

Is Light a Wave or a Particle? Argument: Light is reflected according to the law of reflection, which is a property of waves. Therefore light is a wave.

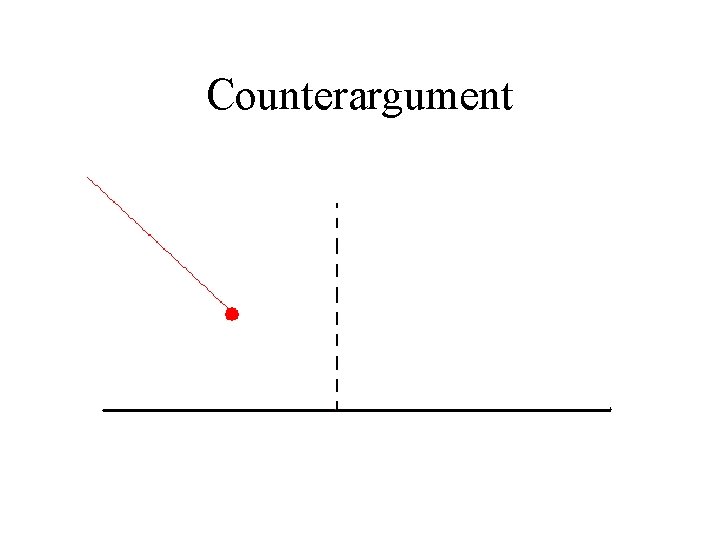
Counterargument

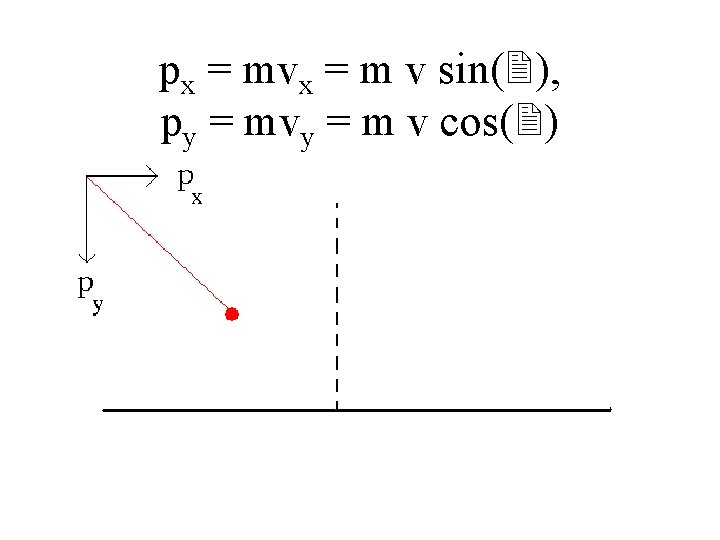
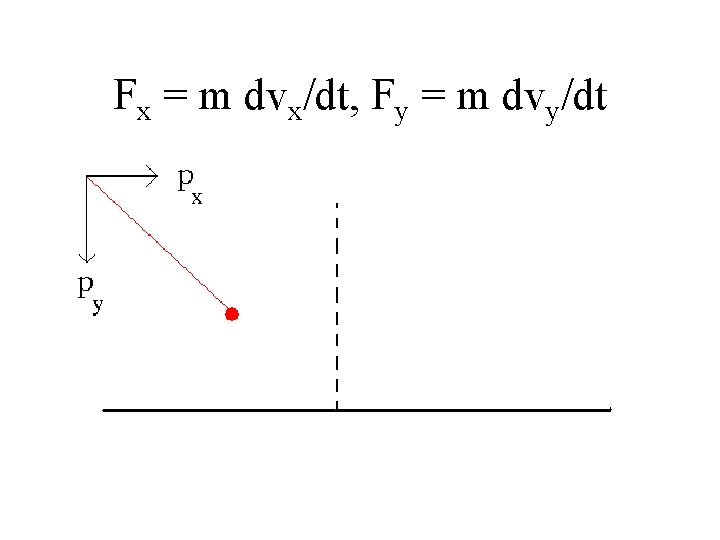
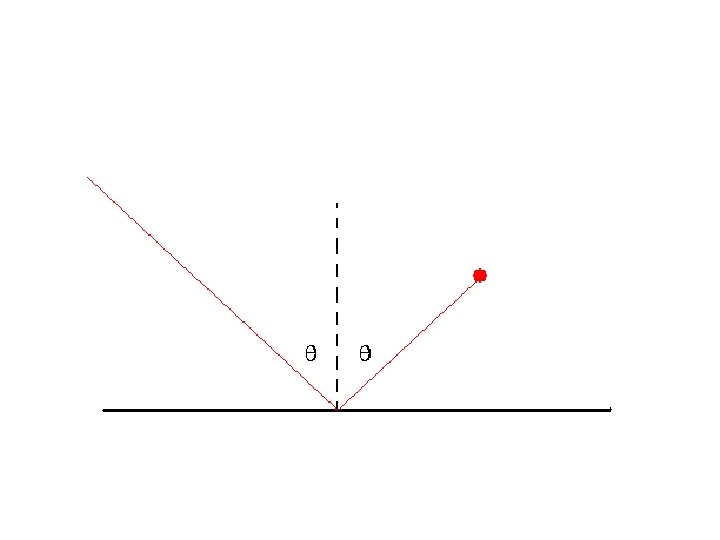
px = mvx = m v sin(2), py = mvy = m v cos(2)

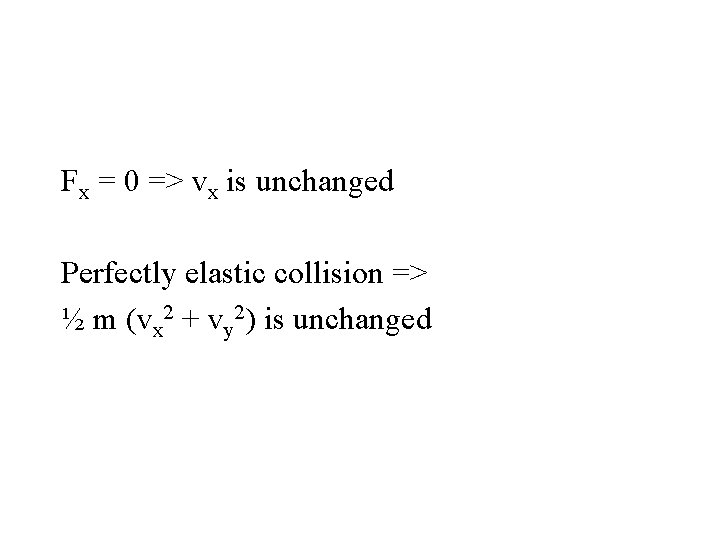
Fx = m dvx/dt, Fy = m dvy/dt

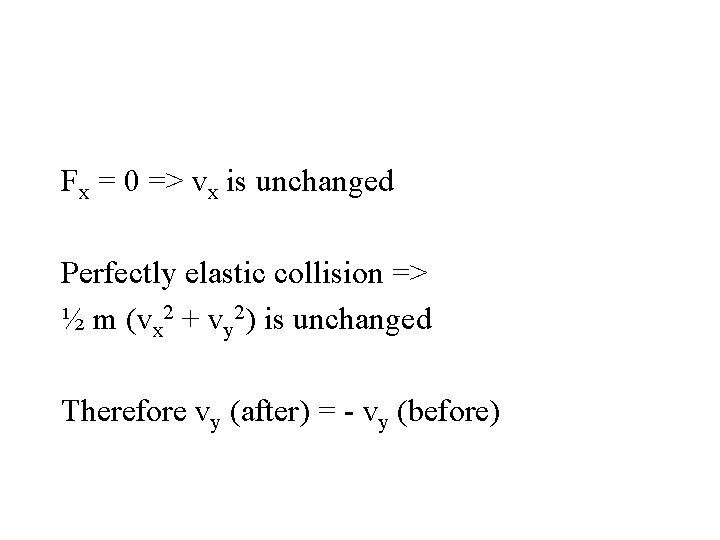
Fx = 0 => vx is unchanged Perfectly elastic collision => ½ m (vx 2 + vy 2) is unchanged

Fx = 0 => vx is unchanged Perfectly elastic collision => ½ m (vx 2 + vy 2) is unchanged Therefore vy (after) = - vy (before)


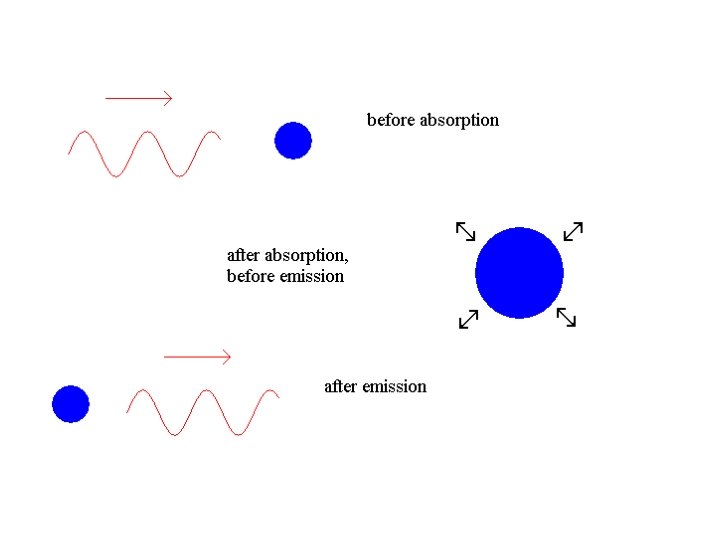
We cannot deduce, simply from the law of reflection, whether light is a wave or a particle.

We cannot deduce, simply from the law of reflection, whether light is a wave or a particle. There is a long history of controversy in optics over whether light is a wave or a particle.

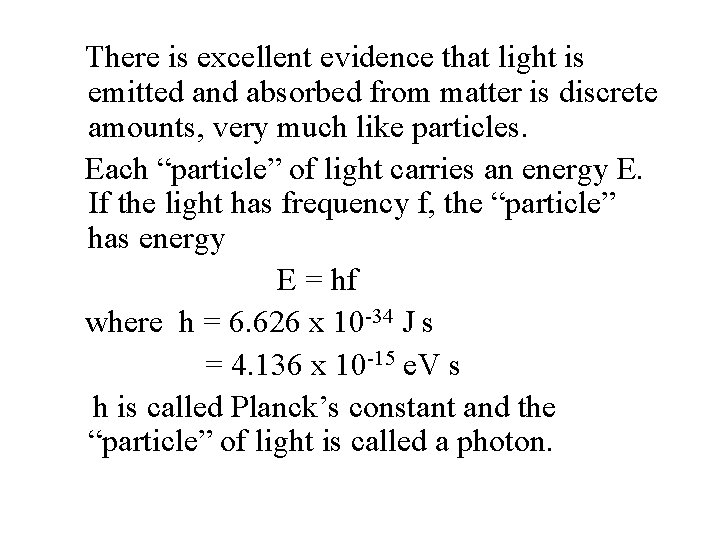
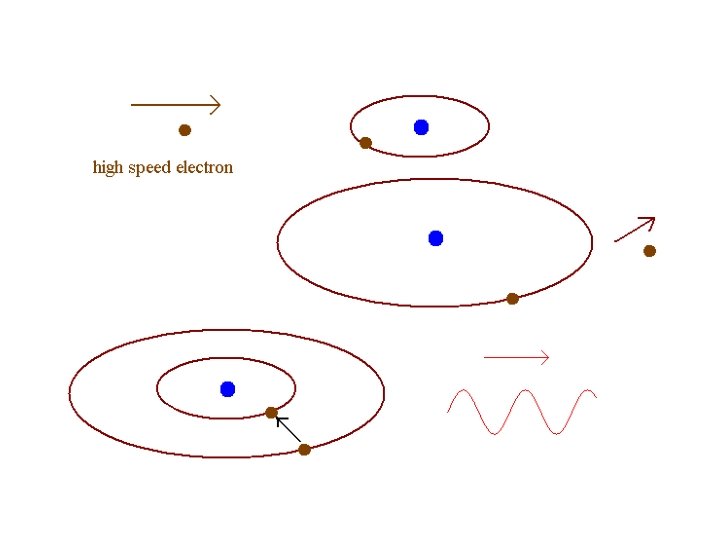
There is excellent evidence that light is emitted and absorbed from matter is discrete amounts, very much like particles. Each “particle” of light carries an energy E. If the light has frequency f, the “particle” has energy E = hf where h = 6. 626 x 10 -34 J s = 4. 136 x 10 -15 e. V s h is called Planck’s constant and the “particle” of light is called a photon.


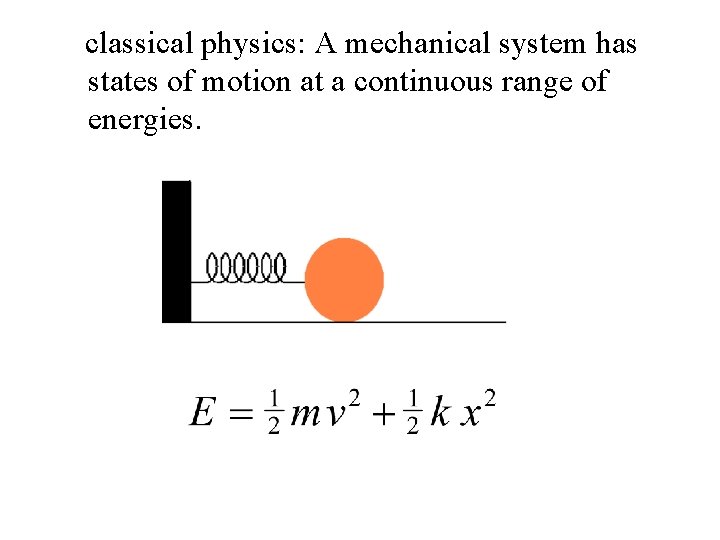
classical physics: A mechanical system has states of motion at a continuous range of energies.

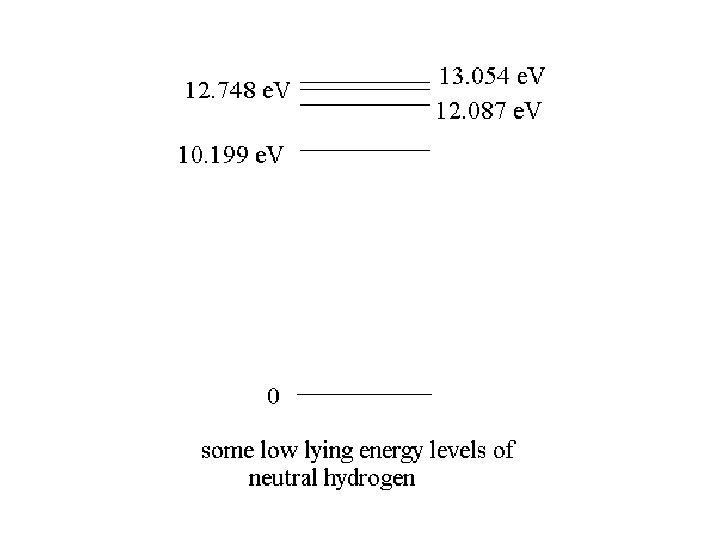
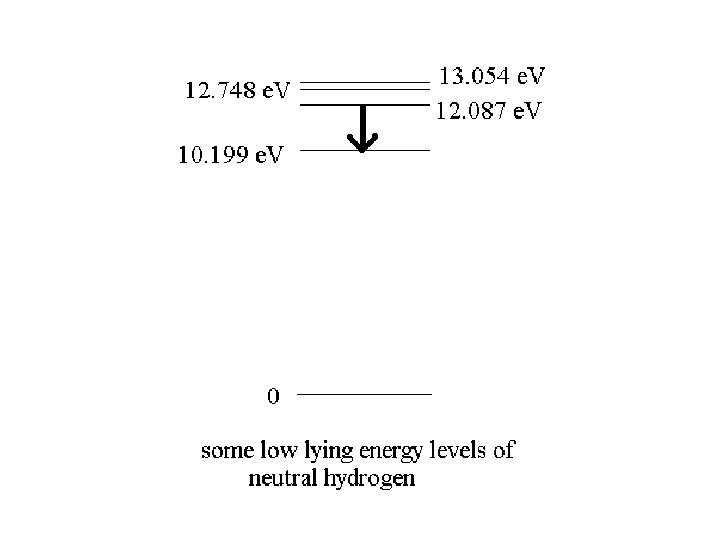
Quantum Physics Atoms and molecules have states of excitation separated by discrete energies



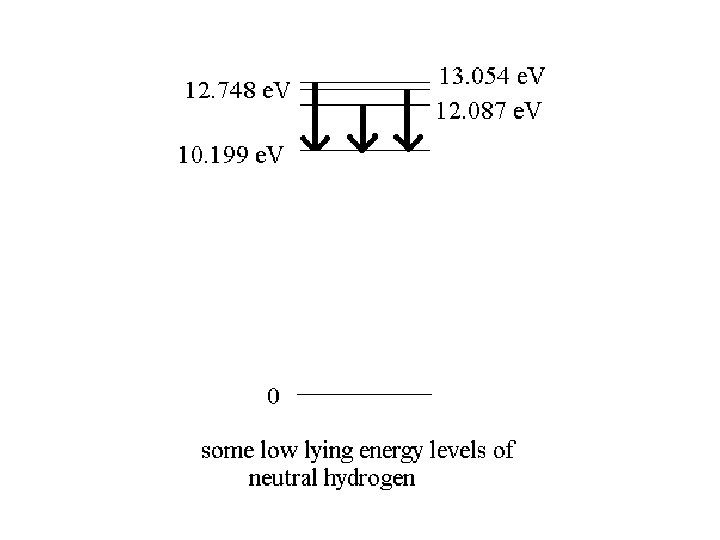
)E = 12. 087 e. V – 10. 199 e. V = 1. 888 e. V The emitted photon has an energy of 1. 888 e. V f = E/h = 1. 888 e. V/ (4. 136 x 10 -15 e. V s) = 4. 565 x 1014 Hz 8 = c / f = (3 x 108 m/s) / (4. 565 x 1014 s-1) = 6. 57 x 10 -7 m = 657 nm

)E = 12. 087 e. V – 10. 199 e. V = 1. 888 e. V The emitted photon has an energy of 1. 888 e. V f = E/h = 1. 888 e. V/ (4. 136 x 10 -15 e. V s) = 4. 565 x 1014 Hz 8 = c / f = (3 x 108 m/s) / (4. 565 x 1014 s-1) = 6. 57 x 10 -7 m = 657 nm The atom emits red light in this transition






)E 1 = 12. 087 e. V – 10. 199 e. V = 1. 888 e. V f 1 = E/h = 1. 888 e. V/ (4. 136 x 10 -15 e. V s) = 4. 565 x 1014 Hz 81= c / f = (3 x 108 m/s) / (4. 565 x 1014 s-1) = 6. 57 x 10 -7 m = 657 nm )E 2 = 2. 549 e. V, )E 3 = 2. 855 e. V 82 = c / f 2 = hc / E 2 = (1240 e. V nm)/ 2. 549 e. V = 486 nm 83 = c / f 3 = hc / E 3 = (1240 e. V nm)/ 2. 855 e. V = 434 nm