Spectroscopy Master Class Spectroscopy Master Class Prepared for











- Slides: 26

Spectroscopy Master Class

Spectroscopy Master Class Prepared for general school use by www. ntu. ac. uk/cels Permission is hereby given to modify the textual content of this file to suit your own classes provided you do not redistribute the work or any derivative version. Please contact CELS if you wish to use either the images or animations in an alternative presentation or related worksheet.

Spectroscopy Aims of this session Mass spectroscopy NMR spectroscopy Back To understand how chemists obtain and interpret the following three types of spectra. IR spectroscopy Next

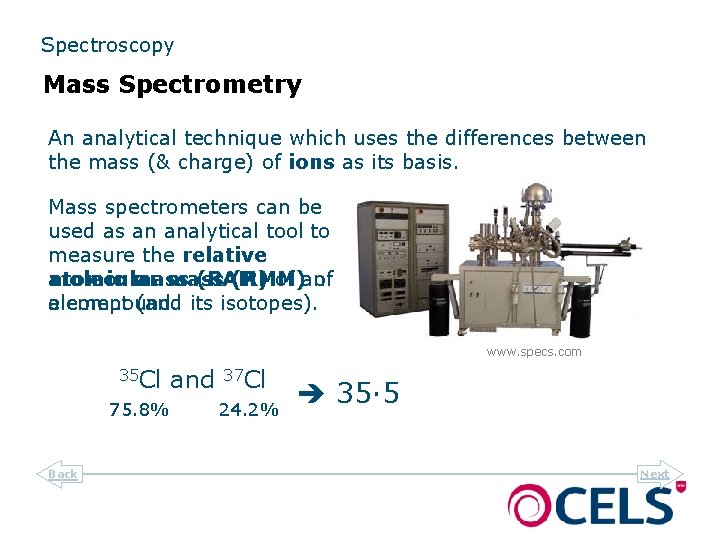
Spectroscopy Mass Spectrometry An analytical technique which uses the differences between the mass (& charge) of ions as its basis. Mass spectrometers can be used as an analytical tool to measure the relative molecular atomic mass (RAM) (RMM) of an of a compound. element (and its isotopes). www. specs. com 35 Cl 75. 8% Back and 37 Cl 24. 2% 35· 5 Next

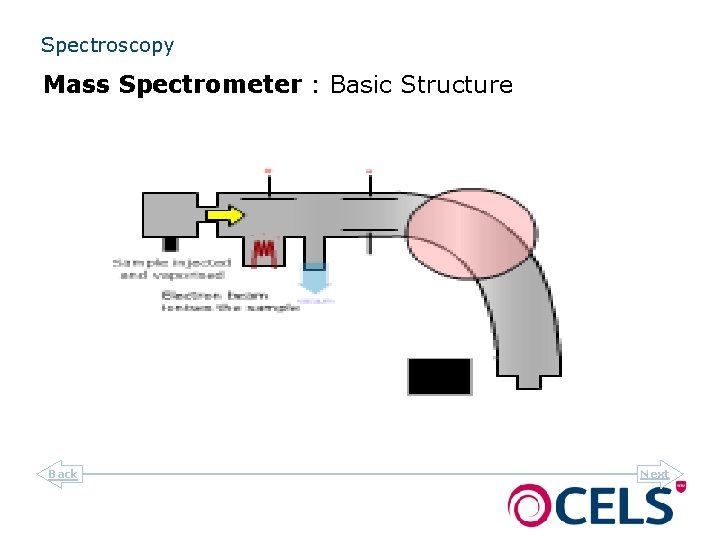
Spectroscopy Mass Spectrometer : Basic Structure Back Next

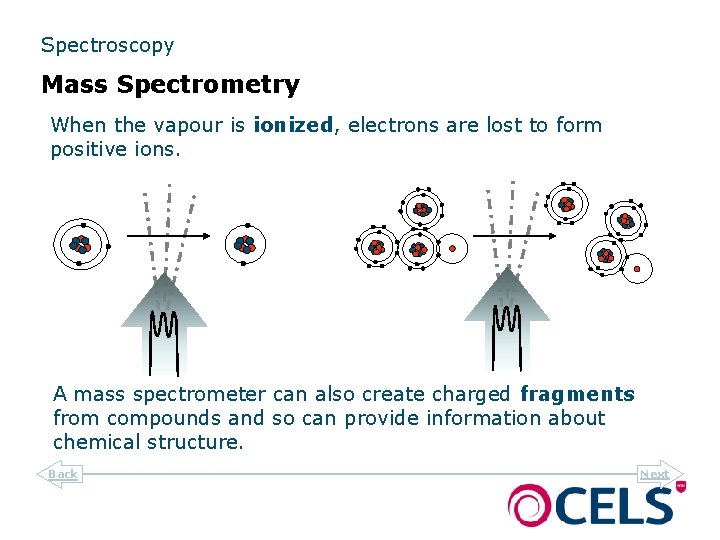
Spectroscopy Mass Spectrometry When the vapour is ionized, electrons are lost to form positive ions. A mass spectrometer can also create charged fragments from compounds and so can provide information about chemical structure. Back Next

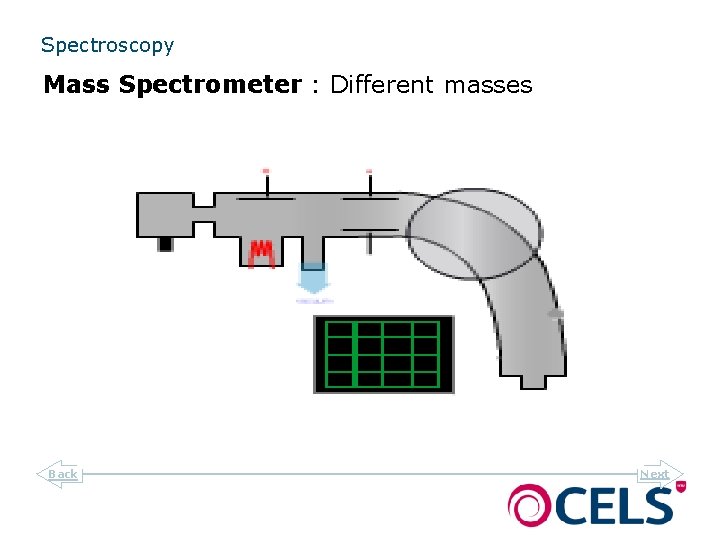
Spectroscopy Mass Spectrometer : Different masses Back Next

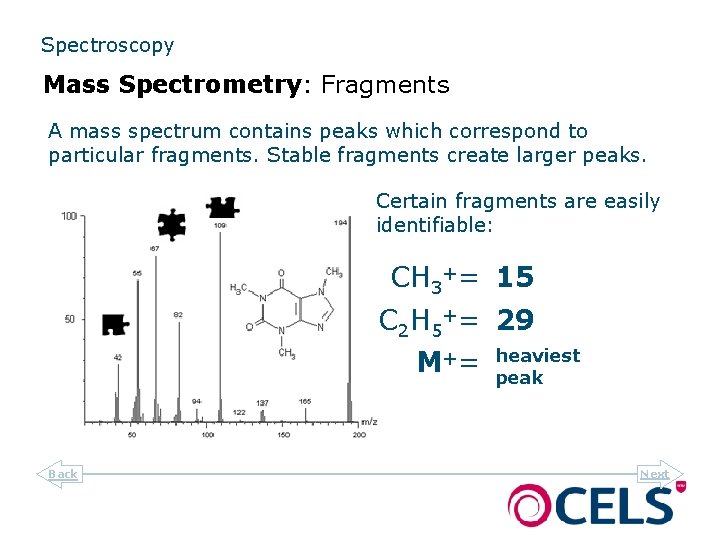
Spectroscopy Mass Spectrometry: Fragments A mass spectrum contains peaks which correspond to particular fragments. Stable fragments create larger peaks. Certain fragments are easily identifiable: CH 3+= 15 C 2 H 5+= 29 M += Back heaviest peak Next

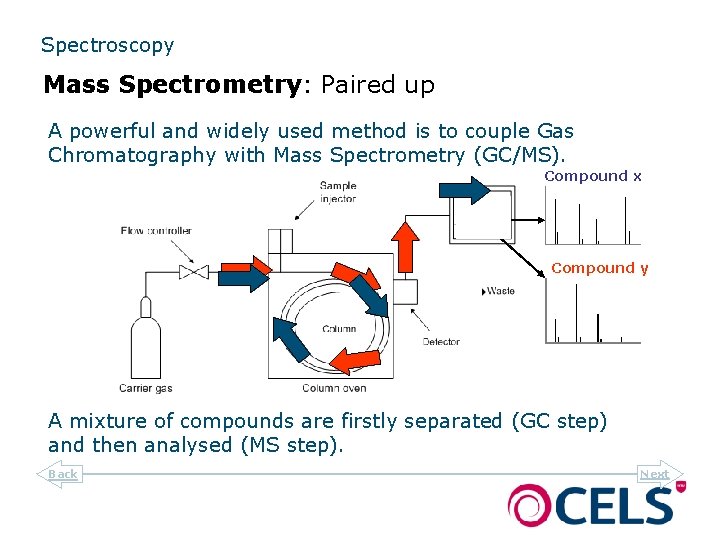
Spectroscopy Mass Spectrometry: Paired up A powerful and widely used method is to couple Gas Chromatography with Mass Spectrometry (GC/MS). Compound x Compound y A mixture of compounds are firstly separated (GC step) and then analysed (MS step). Back Next

Spectroscopy & EMR 1855 Robert Wilhelm Bunsen Back Gustav Kirchhoff Next

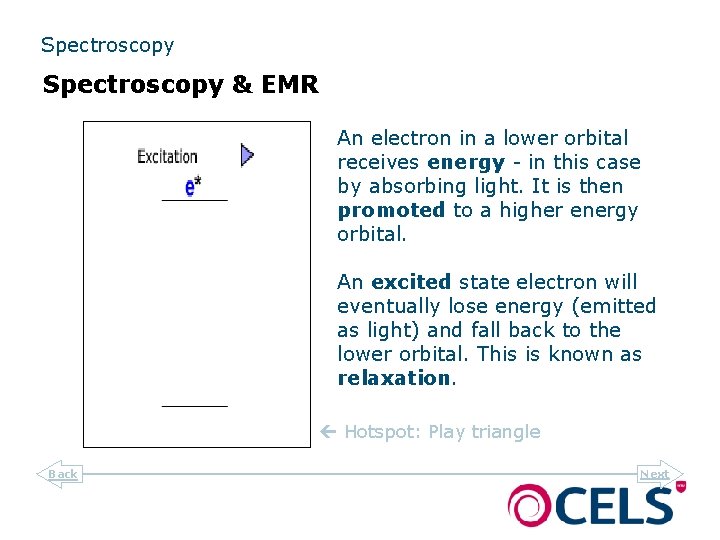
Spectroscopy & EMR An electron in a lower orbital receives energy - in this case by absorbing light. It is then promoted to a higher energy orbital. An excited state electron will eventually lose energy (emitted as light) and fall back to the lower orbital. This is known as relaxation. Hotspot: Play triangle Back Next

Spectroscopy & EMR Emission Absorption Back Next

Spectroscopy Hotspots: Beam, Prism, Slider, Autoscan Back Next

Spectroscopy IR Spectrometry An analytical technique which uses the differences between bonds (& electron levels) as its basis. Bonding electrons absorbing IR cause the bonds to deform. Typical changes to bonds include… Stretching Back Bending Next

Spectroscopy Hotspots: Assigned absorption bands Back Next

Spectroscopy IR Spectrometry Particular useful technique for helping to identify organic functional groups. CH 3 deformation OH bend OH stretch Back CH stretch CO stretch Next

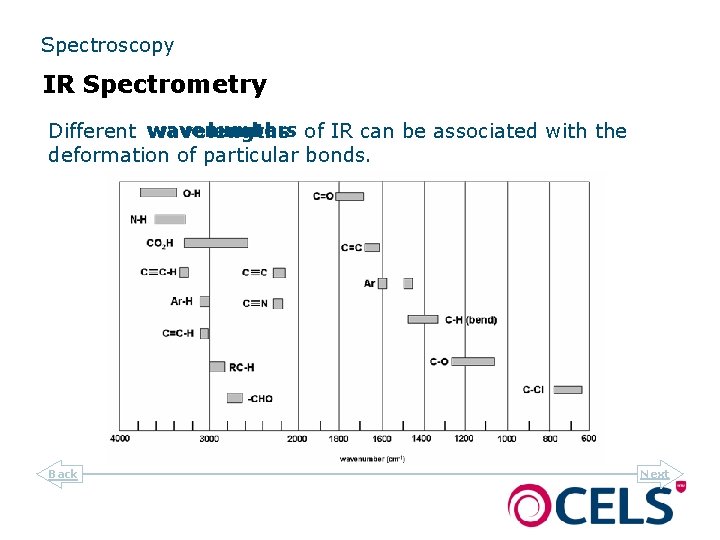
Spectroscopy IR Spectrometry wavenumbers of IR can be associated with the Different wavelengths deformation of particular bonds. Back Next

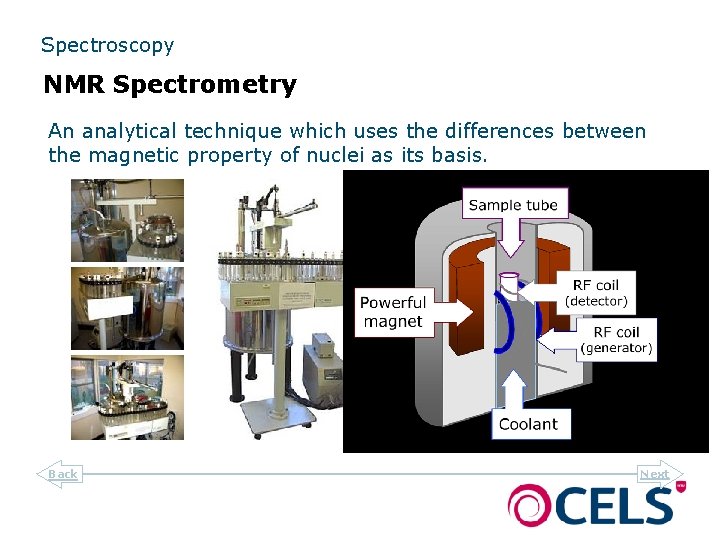
Spectroscopy NMR Spectrometry An analytical technique which uses the differences between the magnetic property of nuclei as its basis. Back Next

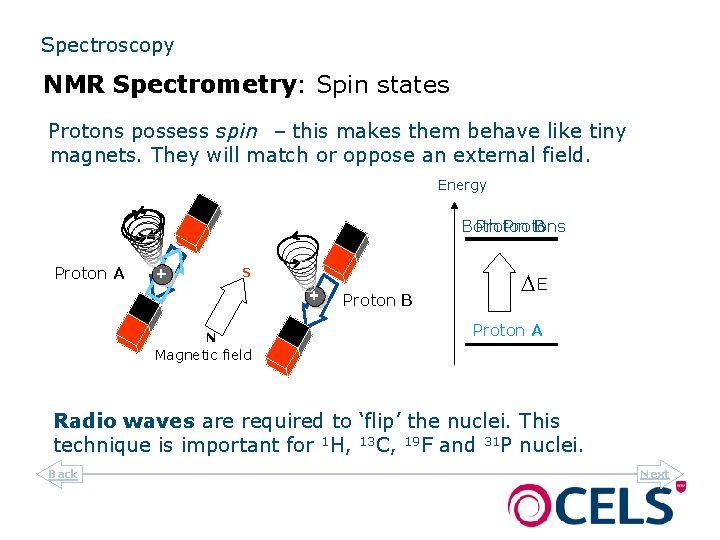
Spectroscopy NMR Spectrometry: Spin states Protons possess spin – this makes them behave like tiny magnets. They will match or oppose an external field. Energy Both Protons Proton B S Proton A S Proton B N N DE Proton A Magnetic field Radio waves are required to ‘flip’ the nuclei. This technique is important for 1 H, 13 C, 19 F and 31 P nuclei. Back Next

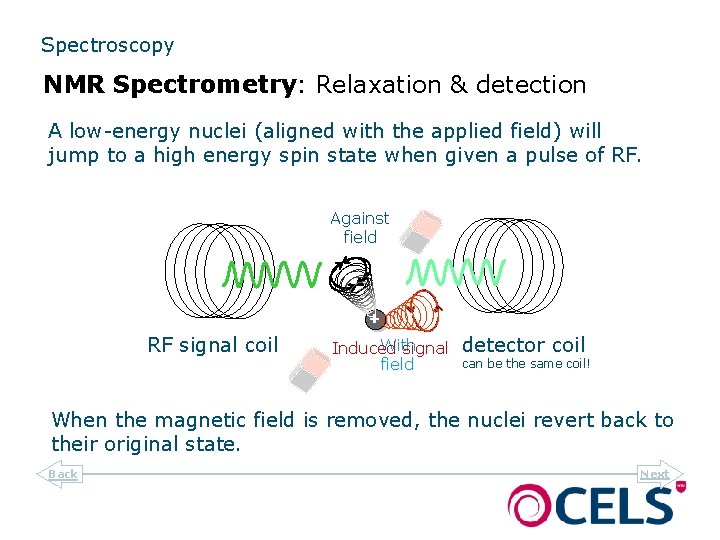
Spectroscopy NMR Spectrometry: Relaxation & detection A low-energy nuclei (aligned with the applied field) will jump to a high energy spin state when given a pulse of RF. Against field RF signal coil With Induced signal field detector coil can be the same coil! When the magnetic field is removed, the nuclei revert back to their original state. Back Next

Spectroscopy NMR Spectrometry: Energy levels The energies of the two spin states relate to the magnetic field. Proton B Weak field Proton B Both protons DE Strong field Proton A We can either fix the field strength and vary the radio waves until the nuclei flip … or… we use one particular radio wave and vary the magnetic field. Back Next

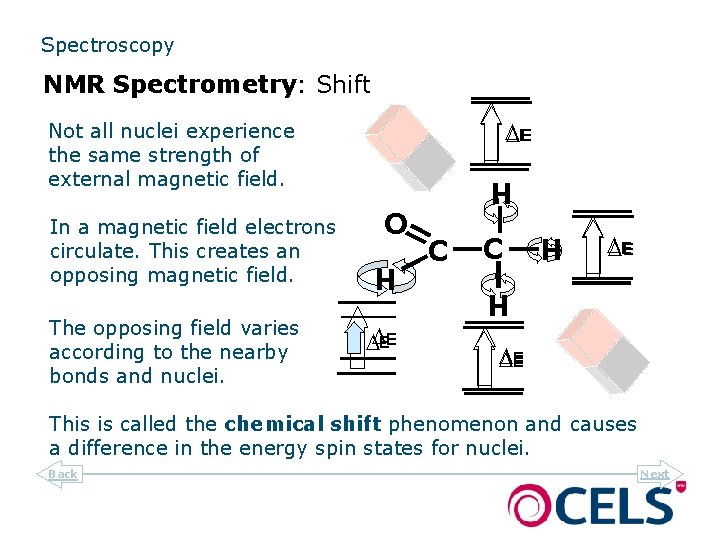
Spectroscopy NMR Spectrometry: Shift DEE Not all nuclei experience the same strength of external magnetic field. C DE H H DDEE C H O The opposing field varies according to the nearby bonds and nuclei. H In a magnetic field electrons circulate. This creates an opposing magnetic field. DE This is called the chemical shift phenomenon and causes a difference in the energy spin states for nuclei. Back Next

Spectroscopy NMR Spectrometry: Assigning peaks C C H H O H The area under each peak relates to the number of each type of hydrogen. H For ethanal, its two types of hydrogen nuclei will produce different signals during NMR. Area = 65 Area = 22 TMS Used to calibrate The signals Back Next

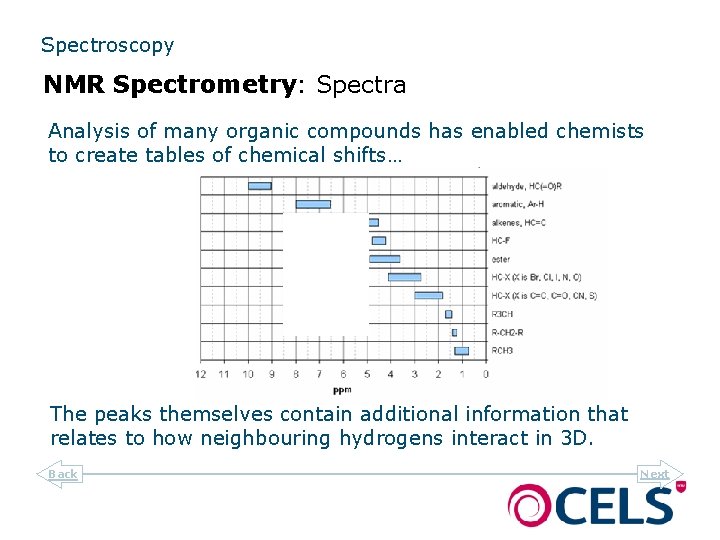
Spectroscopy NMR Spectrometry: Spectra Analysis of many organic compounds has enabled chemists to create tables of chemical shifts… The peaks themselves contain additional information that relates to how neighbouring hydrogens interact in 3 D. Back Next

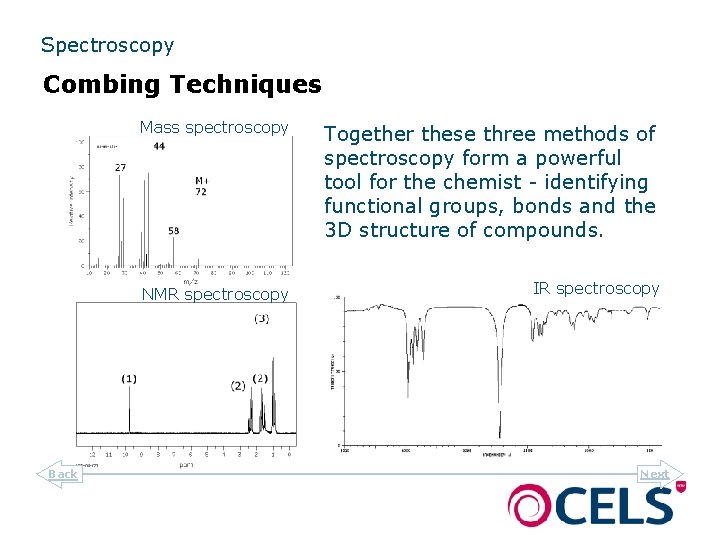
Spectroscopy Combing Techniques Mass spectroscopy NMR spectroscopy Back Together these three methods of spectroscopy form a powerful tool for the chemist - identifying functional groups, bonds and the 3 D structure of compounds. IR spectroscopy Next

Spectroscopy Master Class Prepared for general school use by Centre for Effective Learning in Science www. ntu. ac. uk/cels Back