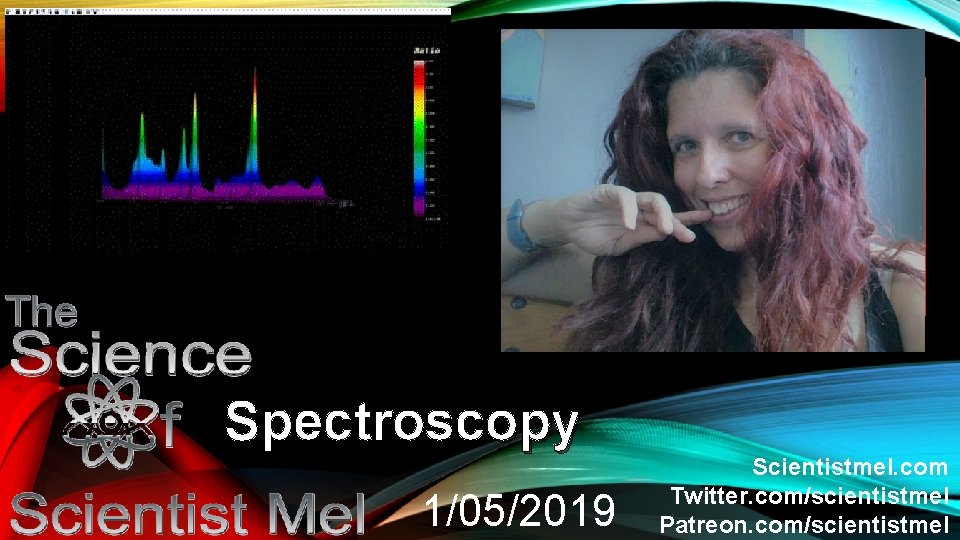
Spectroscopy 1052019 Scientistmel com Twitter comscientistmel Patreon comscientistmel










- Slides: 24

Spectroscopy 1/05/2019 Scientistmel. com Twitter. com/scientistmel Patreon. com/scientistmel

SPECTROSCOPY • Types of Spectroscopy • How it works • Difference from Mass Spec

SPECTROSCOPY Theory • EM Based • Absorption • Emission

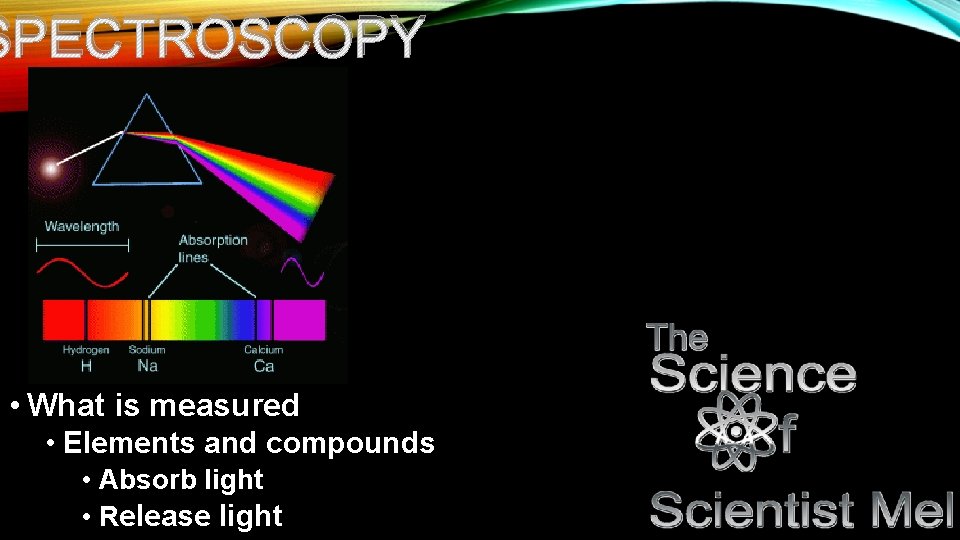
SPECTROSCOPY • What is measured • Elements and compounds • Absorb light • Release light

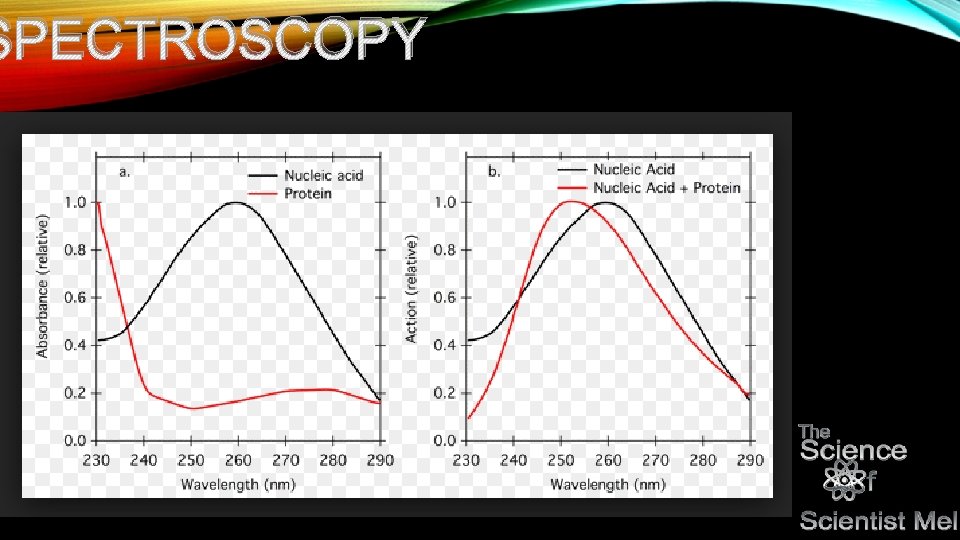
NP • Specific wavelengths of light…uv-visible spectrum excite electrons as they hit elements and compounds. Only specific wavelengths excite classes of compounds. Because these wavelengths are so specific to these compounds, they make for a great way to detect them in solutions to determine their presence. • For example, proteins tend to absorb at the 280 nm wavelength. Genetic code tends to hit at the 260 nm wavelength…this is largely due to their structures. • It is also important to note that the type of bond involved with the compound determines what wavelength it absorbs. • Double bonds that are conjugated (meaning every other bond is a double bond) absorb light in the UV spectrum. The more a conjugated system, the stronger the presence in the UV spectrum…thus we use sunscreen that absorbs in the UV spectrum in order to fight sunburns as melanin (what makes our skin darker) can only withstand so much UV radiation.

NP • Infrared differs from UV-Vis in that it measures vibrations not absorption. If you remember from my photon talk, if you haven’t checked that out, it helps a bit with this one…infrared waves give off heat…this is due to causing atoms to vibrate…and infrared spectroscopy measures vibrations at particular wavelengths. Certain compounds show up in the infrared spectrum…one particular use for infrared (IR) is nanoparticle tech (a talk I gave last month and you can check it out here!) Metal based nanoparticles have strong infrared signals. So, you can bind proteins to nanoparticles to enhance their IR signal. This is particularly handy in that you can specialize nanoparticles to target particulary bad cells like cancer in a solution with very high sensitivity and accuracy. • We are going to look at a few specs in just a bit…I will focus mostly on Uvvis…and we will check out what a box diagram of the instrument looks like…But first let’s talk about MATH

SPECTROSCOPY Beers Law • • Absorbance (A) Path length (b) Extinction coefficient Concentration (c)

NP • Remember when I said that proteins hit at 280 nm wavelength and DNA hits at 260 nm wavelength? Let’s say that you have a solution that you are trying to determine the concentration of a protein and DNA within it…you go over to a UV -Vis spec instrument and place your sample in a 1 cm cuvette. • You push the button. You get a reading (number) and a curve. • Now let’s see what the curves look like…

SPECTROSCOPY

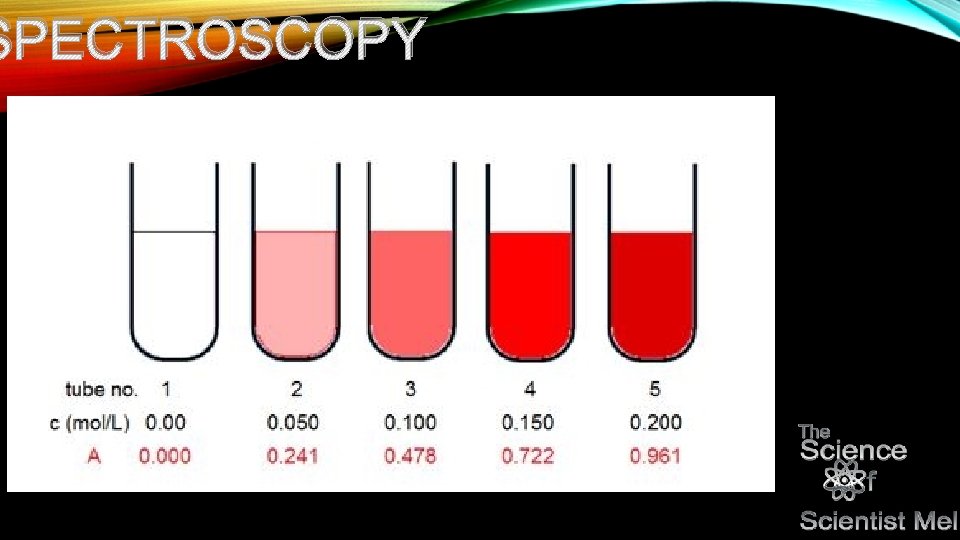
NP Let’s say you want to make different concentrations of this solution to double check your concentration…So, you go through and do what is called a serial dilution…starting from full concentration to 75%, 50%, 25%, to 0%. Let’s say that you ru your sample again looking for protein. What would that look like?

SPECTROSCOPY

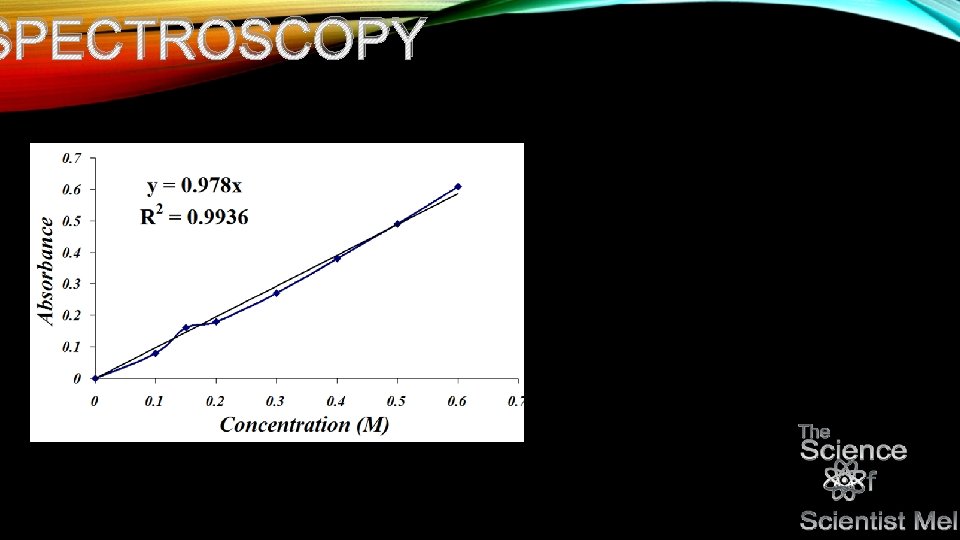
NP • You can then take this data, plot it on a graph and determine the concentration based on good old y=mx+b…you got it! Algebra! Once you get your equation

SPECTROSCOPY

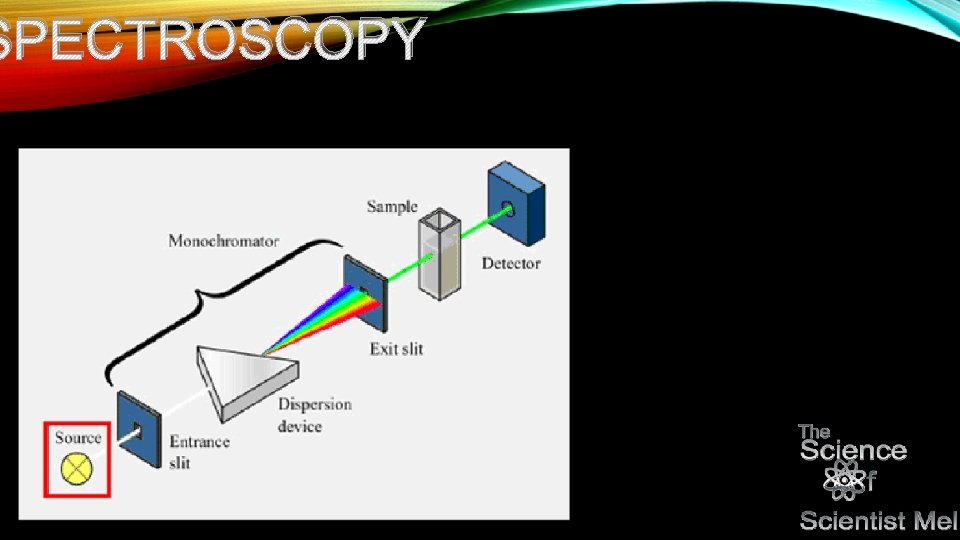
NP • it is important to note that spectrometers tell you which wavelengths of light were absorbed…a spectrophotometer measures the intensity of the light absorbed or reflected at a wavelength. • Let’s take a look at box diagrams for specs…

SPECTROSCOPY

NP • Monochromators can be tweaked to be selective to specific wavelengths…the amplifier essentially strengthens the signal…one particular amplifier is the photo multiplier tube that can increase the signal from the solution through exciting other electrons. • Light sources can vary and it depends on whether you are using infrared or UV -Vis…The intensity at specific wavelengths is important infrared has longer wavelengths while UV-vis has shorter…thus the correct bulb is important. • You can also have a thing called noise…it can affect your solution…background noise happens for various reasons…your sample may not be as pure as you think. If your noise is as strong as your signal, that’s not going to give you a very good reading…this is where cleaning up your sample comes into play…but that is another talk.

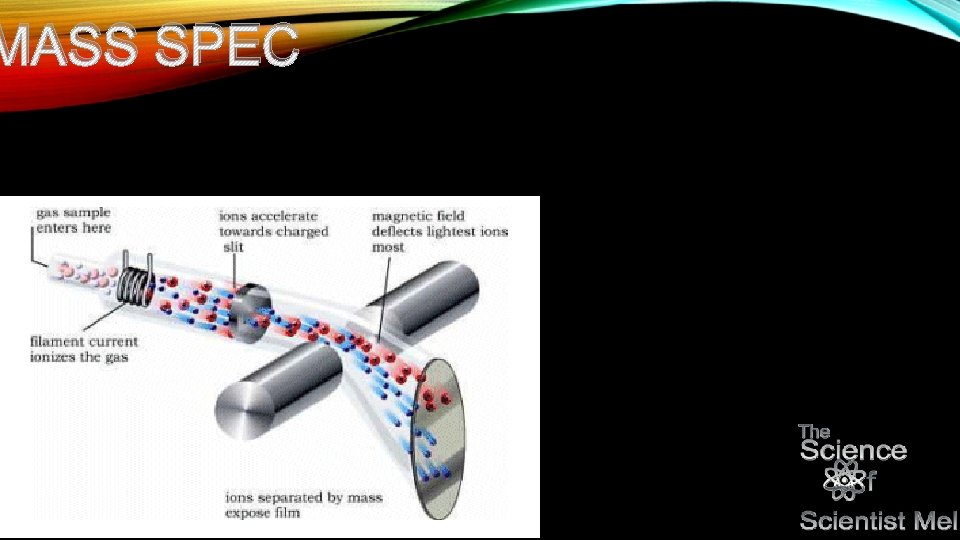
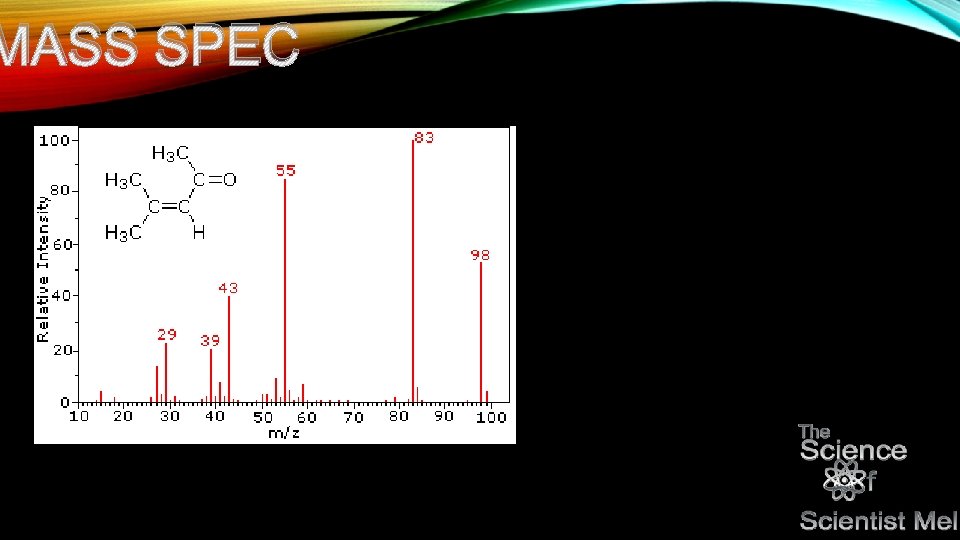
KETO • Mass spec is completely different from spectroscopy involving electromagnetic radiation… • Discuss time of flight and quadropoles…get into fraction patterns. Tells what functional groups are there based on breaking patterns. • Can be used in coordination with em spec to finalize functional groups and also prior to mass spec to determine type of compound.

MASS SPEC

MASS SPEC

SPECTROSCOPY • Types of Spectroscopy • How it works • Difference from Mass Spec

• • THANK YOU TO MY • Paola PATRONS • Graham James • Andy • Keri Jenn • Circe Carl • Keith Melanie • Duke Patrick • James Daniel Steven • Zachary • Dragnaucht • Godless Iowan Tony • Bo • Jennifer • Steven • Richard • Sarah • Doc Fearsome • Chris • Neil •

You can find me… • • • Scientist. Mel. com Patreon. com/scientistmel Pscp. tv. com/scientistmel Youtube. com/scientistmel Facebook. com/scientistmel

Spectroscopy 1/05/2019 Scientistmel. com Twitter. com/scientistmel Patreon. com/scientistmel

• SOURCES • AZONano. com • https: //www. aranca. com/knowledge-library/infographics/ip-research/types-ofprevalent-nanoparticles • https: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 3865110/