Spectrophotometry Xray Fluorescence XRF Xray Fluorescence Spectrophotometry is










- Slides: 17

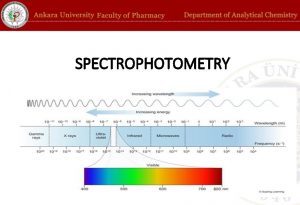
Spectrophotometry X-ray Fluorescence

XRF • X-ray Fluorescence Spectrophotometry is a non-destructive method in which samples are bombarded with X-ray radiation and the residual X-rays that fluoresce are measured.

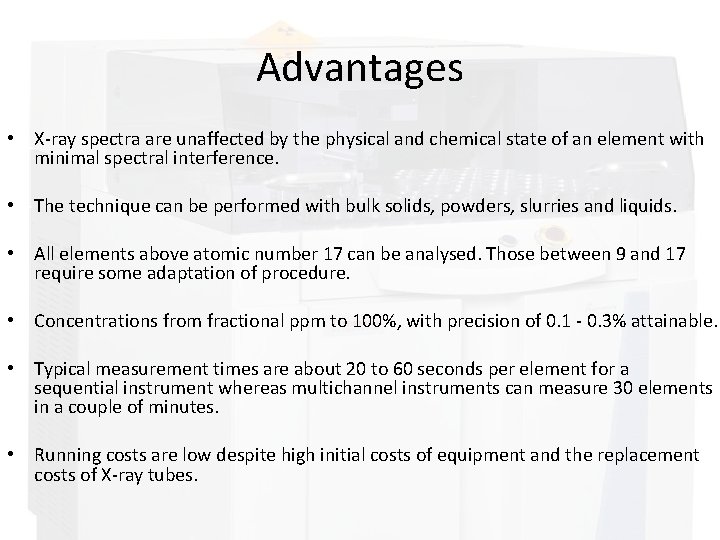
Advantages • X-ray spectra are unaffected by the physical and chemical state of an element with minimal spectral interference. • The technique can be performed with bulk solids, powders, slurries and liquids. • All elements above atomic number 17 can be analysed. Those between 9 and 17 require some adaptation of procedure. • Concentrations from fractional ppm to 100%, with precision of 0. 1 - 0. 3% attainable. • Typical measurement times are about 20 to 60 seconds per element for a sequential instrument whereas multichannel instruments can measure 30 elements in a couple of minutes. • Running costs are low despite high initial costs of equipment and the replacement costs of X-ray tubes.

Disadvantages • The inability to measure elements below atomic number 9. • Because of the short penetration power of X-rays, there is a need for homogeneous sample and standard preparation to minimise the effects of surface texture and particle size. • More sensitive methods are available through ICP-OES and ICP-MS. • In spite of rapid analysis time the preparation of samples, standards and implementation of standard addition is time consuming. • Standards need to be similar to unknowns in all respects, which may be hard to achieve. • XRF analysis requires highly trained staff to operate the equipment.

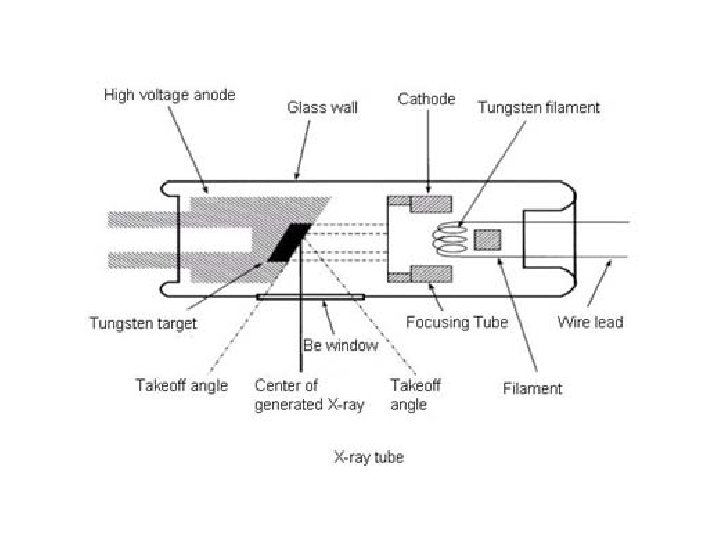
Components • An X-ray spectrometer consists of five main components: – The X-ray tube – The specimen chamber – The collimators – The crystal – The detector




X-ray Spectra

X-ray Spectra




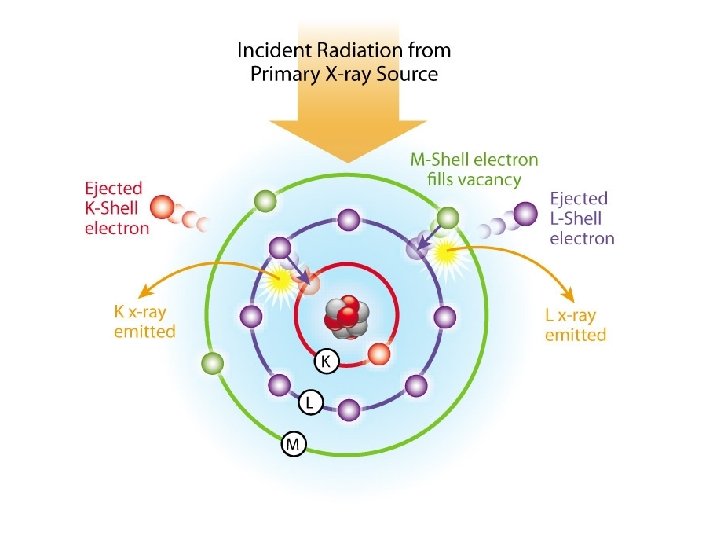
Crystal • Is used as a diffraction grating for the analysis which disperses the X-ray beam so it can be read by the detector.

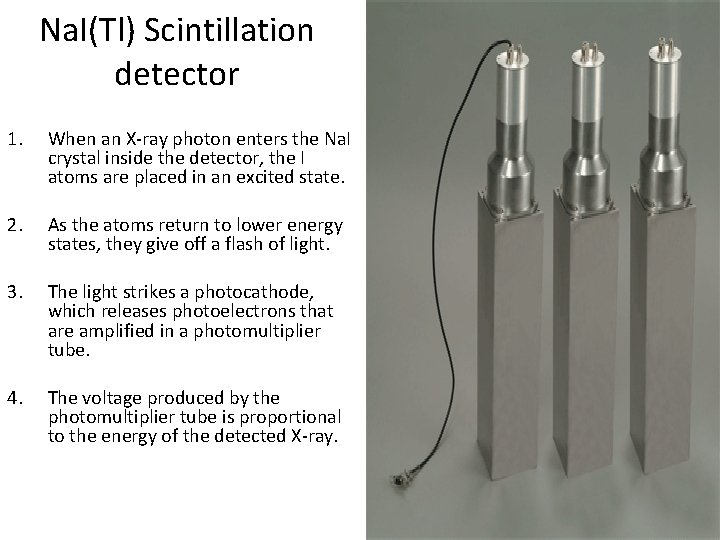
Na. I(Tl) Scintillation detector 1. When an X-ray photon enters the Na. I crystal inside the detector, the I atoms are placed in an excited state. 2. As the atoms return to lower energy states, they give off a flash of light. 3. The light strikes a photocathode, which releases photoelectrons that are amplified in a photomultiplier tube. 4. The voltage produced by the photomultiplier tube is proportional to the energy of the detected X-ray.


Applications Soil surveys Mining – Iron Ore Cement production Ceramic and Glass Manufacturing Metallurgy Petroleum Industry
 Xrf theory ppt
Xrf theory ppt Shimadzu edx-720
Shimadzu edx-720 Niton xl 800
Niton xl 800 Micro pioneer xrf 2000
Micro pioneer xrf 2000 Analzy
Analzy Xrf çalışma prensibi
Xrf çalışma prensibi Xrf method
Xrf method Proteus xrf
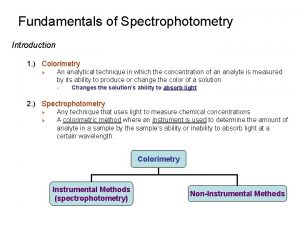
Proteus xrf Introduction to colorimetry
Introduction to colorimetry Biotechnology topics
Biotechnology topics Atomic absorption spectrophotometer principle
Atomic absorption spectrophotometer principle Lambert's law
Lambert's law Readout device
Readout device Introduction to spectrophotometry
Introduction to spectrophotometry Nephelometry
Nephelometry Beer's law equation example
Beer's law equation example Introduction to spectrophotometry
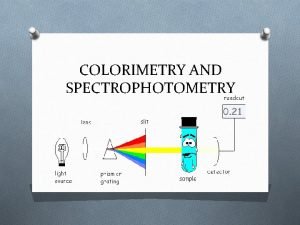
Introduction to spectrophotometry Colorimetry and spectrophotometry
Colorimetry and spectrophotometry