Spectrophotometric Methods Part 1 Dependence of Absorbance on
Spectrophotometric Methods Part 1: Dependence of Absorbance on Concentration Dr. Prem D. Sattsangi Christopher L. Byers (programmer) © 2009

Spectrophotometric Studies • Aparatus: • Spectronic 20 Spectrophotometer (Spec-20) • Blank with DW, and Kimwipes • Procedure: • On Spec-20, adjust wavelength to 450 nm. • Wipe the Blank Cuvet clean and insert it in Spec-20 to set zero.
Absorbance VS. Wavelength of KMn. O 4 • Procedure: Insert KMn. O 4 solution. Record Absorbance at 425 nm. Adjust wavelength to 475 nm. Wipe the Blank Cuvet clean and insert it in Spec-20 to set zero. • Insert KMn. O 4 solution. • Record Absorbance. • Continue recording absorbance at 25 nm intervals up to 700 nm. • • In the region of the λmax select ± 25 nm to record absorbance at 5 nm intervals. Plot absorbance vs. wavelength graph.

Visible (400 - 800 nm) spectrum for KMn. O 4 Lambda max, λmax= 525 nm
Prepare a Beer’s Law plot (Absorbance VS. Molar concentration) • Procedure: • On Spec-20, adjust wavelength to λmax. • Prepare solutions having a ratio of 4. 00 x 10 -4 M KMn. O 4 solution to distilled water of 1: 3 (1. 00 x 10 -4 M), 2: 2 (2. 00 x 10 -4 M), & 3: 1 (3. 00 x 10 -4 M). • Measure the absorbance of each dilution at λmax. • Prepare graph, Beer’s Law Plot, using each absorbance reading.
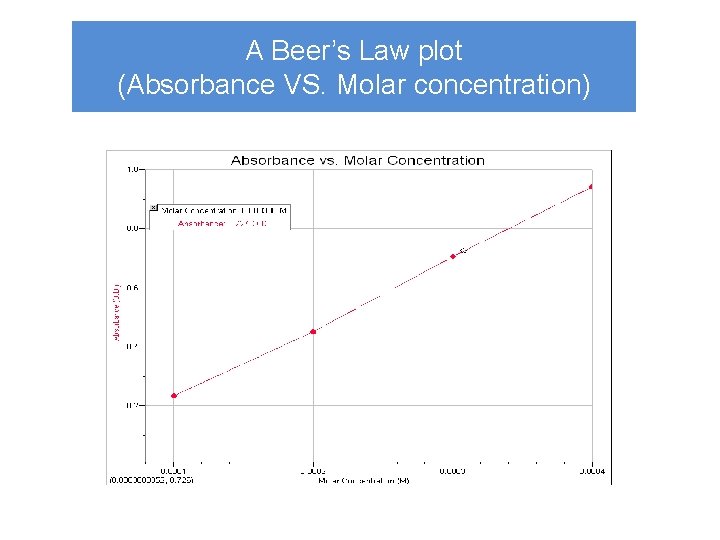
A Beer’s Law plot (Absorbance VS. Molar concentration)
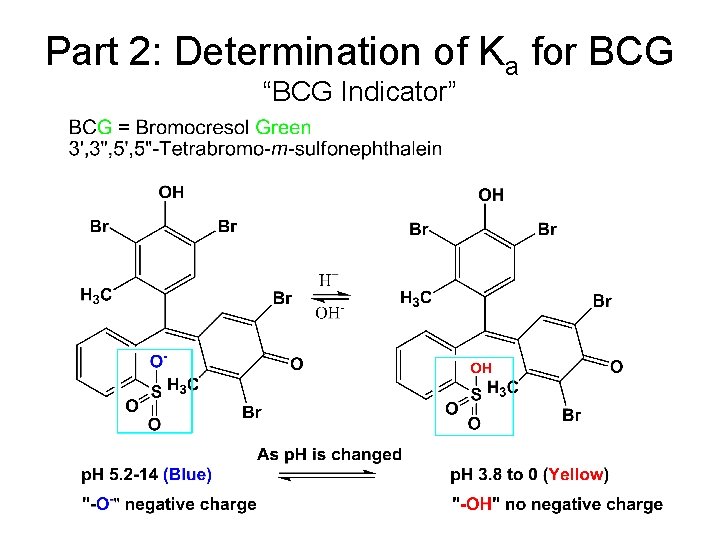
Part 2: Determination of Ka for BCG “BCG Indicator”

Solutions A and 1 • Solution-A: supplied is 25. 00 m. L of 1. 00 M HOAc + 5. 00 m. L of 3. 00 x 10 -4 M BCG diluted to 100 m. L. • Solution-1: to be made by you by mixing 5. 00 m. L each of 0. 200 M Na. OAc and 3. 0 x 10 -4 M BCG. These solutions are available in 25 m. L Burets. ACIDIC BASIC

Points to Remember • Never discard any of your solutions. • Use a disposable pipet to transfer solution from cuvet to flask and vice versa. • Always handle cuvet with kimwipes, never with bare hands. • Use a 50 m. L beaker to store your cuvets when not in use. • Always remember to zero the Spec-20, with the blank cuvet, after changing the wavelength. 0

Absorbance of Solution-1 Procedure: Take Solution-1 in Sample cuvet. Remove the blank from Spec-20. Place Sample cuvet in Spec-20. Record Absorbance from 450 nm – 650 nm, at 25 nm intervals. • [Each reading has to begin by: a) selecting the new wavelength b) zeroing with the blank c) recording the absorbance of the sample. ] • Near the λmax absorption is recorded at 5 nm intervals (3 above λmax and 3 below) to find a more accurate λmax. • Transfer Solution-1 from cuvet back to Erlenmeyer flask. • • •

Making Solution-2 • Procedure: • Pour Solution-1 into 250 m. L Erlenmeyer. • Using an auto-pipet, take 2 m. L of Solution-A and add to Erlenmeyer containing Solution-1 to make Solution-2. • [The blue color changes to green. ]

Absorbance of Solution-2 and Making Solution-3 • Procedure: • Rinse sample cuvet with Solution 2 (without discarding). • Take Solution-2. • Place sample in Spec-20. • Record Absorbance Reading at λmax (determined earlier). • Transfer Solution-2 from cuvet back to Erlenmeyer flask. • Using pipet, take 2 m. L of Solution A, add to Erlenmeyer containing Solution-2 to make Solution-3.

Absorbance of Solution-3 and Making Solution-4 • Procedure: • Rinse sample cuvet with Solution 3 (without discarding). • Take Solution-3. • Place sample in Spec-20. • Record Absorbance Readings from 400 nm – 650 nm. • Transfer Solution-3 from cuvet back to Erlenmeyer flask. • Using pipet, take 2 m. L of Solution A, add to Erlenmeyer containing Solution-3 to make Solution-4.

Absorbance of Solution-4 and Making Solution-5 • Procedure: • Rinse sample cuvet with Solution 4 (without discarding). • Take Solution-4. • Place sample in Spec-20. • Record Absorbance Reading at λmax (determined earlier). • Transfer Solution-4 from cuvet back to Erlenmeyer flask. • Using pipet, take 2 m. L of Solution A, add to Erlenmeyer containing Solution-4 to make Solution-5.

Absorbance of Solution-5 and Making Solution-6 • Procedure: • Rinse sample cuvet with Solution 5 (without discarding). • Take Solution-5. • Place sample in Spec-20. • Record Absorbance Reading at λmax (determined earlier). • Transfer Solution-5 from cuvet back to Erlenmeyer flask. • Using pipet, take 2 m. L of Solution A, add to Erlenmeyer containing Solution-5 to make Solution-6.

Absorbance of Solution-6 and Making Solution-7 • Procedure: • Rinse sample cuvet with Solution 6 (without discarding). • Take Solution-6. • Place sample in Spec-20. • Record Absorbance Reading at λmax (determined earlier). • Transfer Solution-6 from cuvet back to Erlenmeyer flask. • Using pipet, take 1 m. L of 3 M HCl/BCG, add to Erlenmeyer containing Solution-6 to make Solution-7.
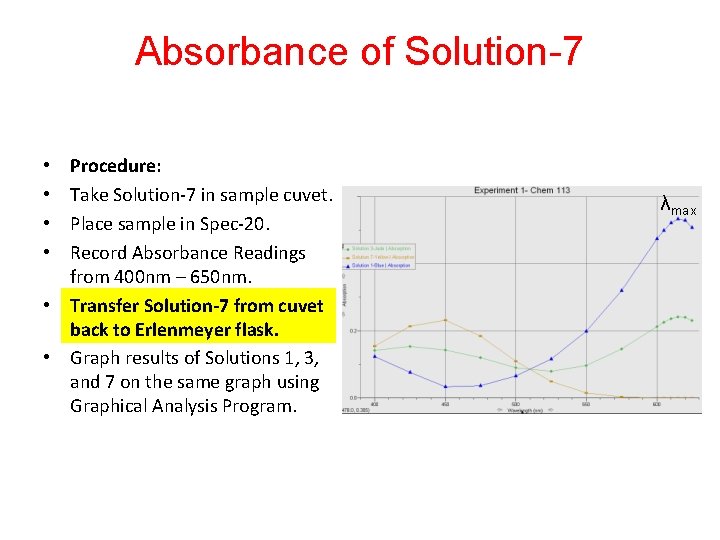
Absorbance of Solution-7 Procedure: Take Solution-7 in sample cuvet. Place sample in Spec-20. Record Absorbance Readings from 400 nm – 650 nm. • Transfer Solution-7 from cuvet back to Erlenmeyer flask. • Graph results of Solutions 1, 3, and 7 on the same graph using Graphical Analysis Program. • • λmax
- Slides: 17