Spectrally Identifiable Functional Groups The Mass Spectrometer A


Spectrally Identifiable Functional Groups

The Mass Spectrometer A mass spectrum records only positively charged fragments, either cations or radical cations m/z = mass-to-charge ratio of the fragment 3

An Example of a Mass Spectrometer
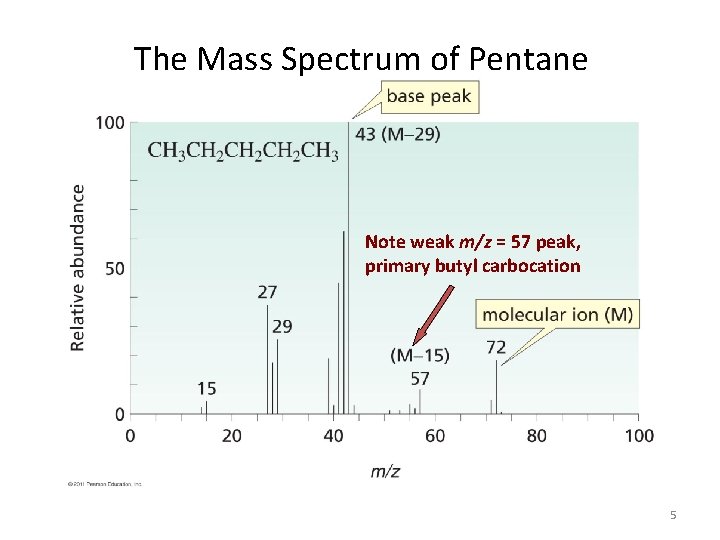
The Mass Spectrum of Pentane Note weak m/z = 57 peak, primary butyl carbocation 5
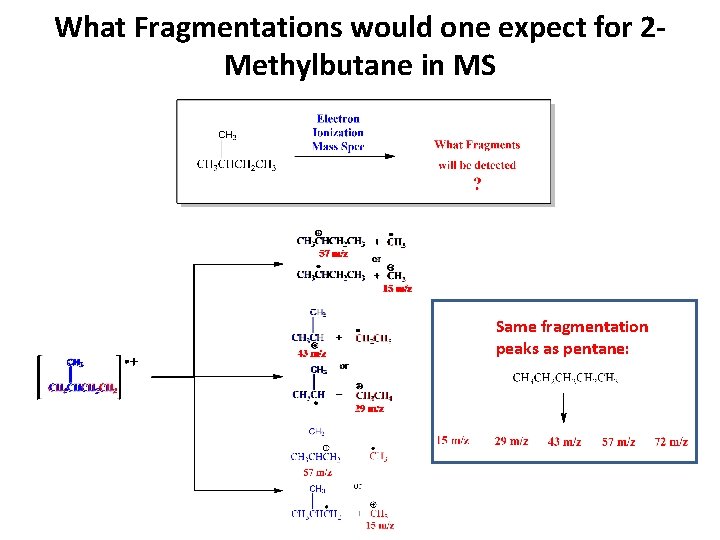
What Fragmentations would one expect for 2 Methylbutane in MS Same fragmentation peaks as pentane:
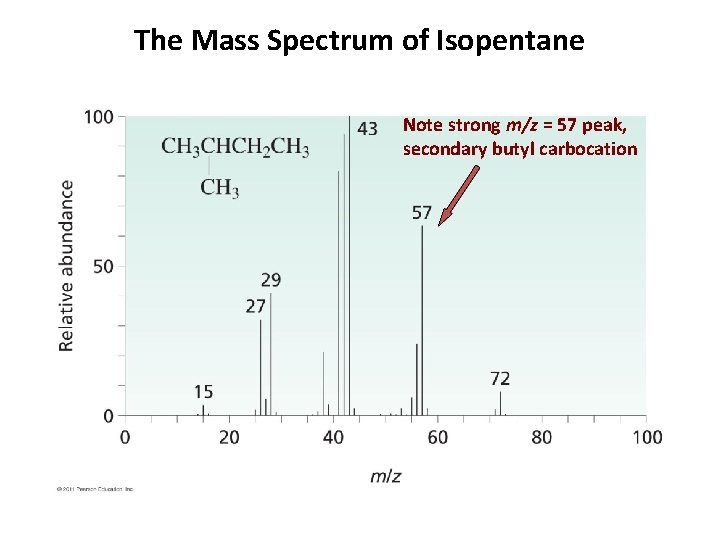
The Mass Spectrum of Isopentane Note strong m/z = 57 peak, secondary butyl carbocation
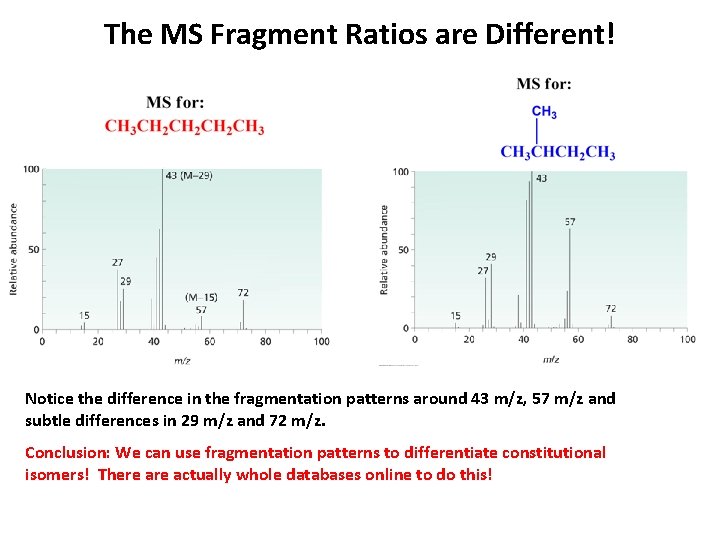
The MS Fragment Ratios are Different! Notice the difference in the fragmentation patterns around 43 m/z, 57 m/z and subtle differences in 29 m/z and 72 m/z. Conclusion: We can use fragmentation patterns to differentiate constitutional isomers! There actually whole databases online to do this!
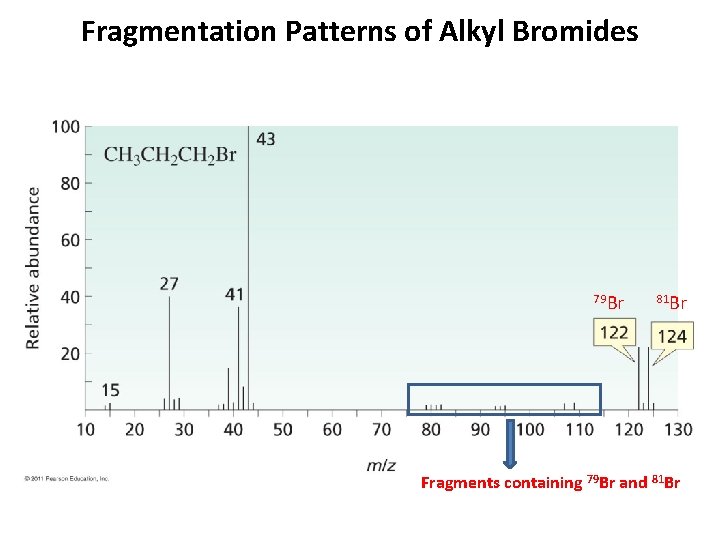
Fragmentation Patterns of Alkyl Bromides 79 Br 81 Br Fragments containing 79 Br and 81 Br
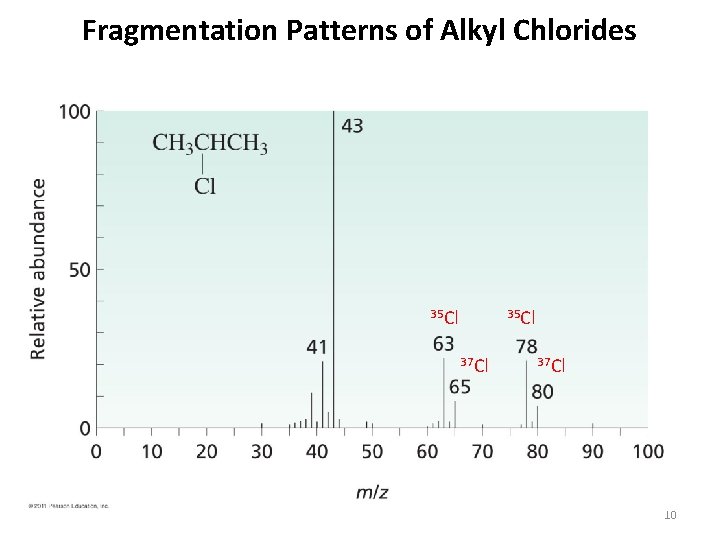
Fragmentation Patterns of Alkyl Chlorides 35 Cl 37 Cl 10
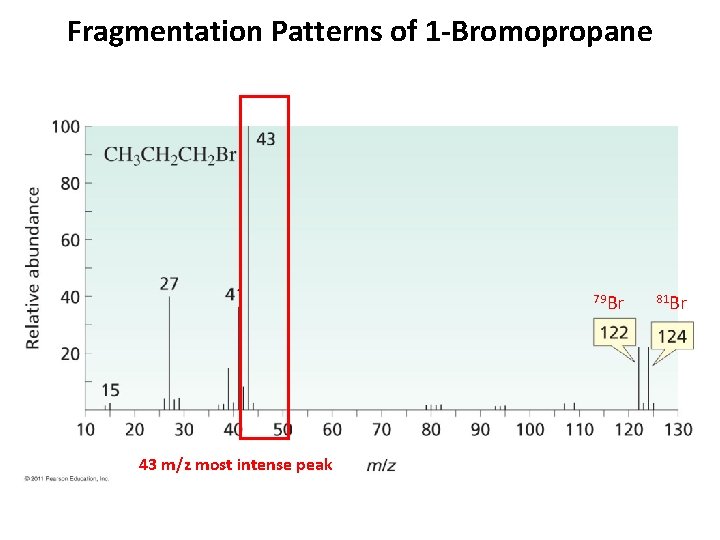
Fragmentation Patterns of 1 -Bromopropane 79 Br 43 m/z most intense peak 81 Br

Fragmentation Patterns of Alkyl Chlorides 43 m/z most intense peak 35 Cl 37 Cl 12
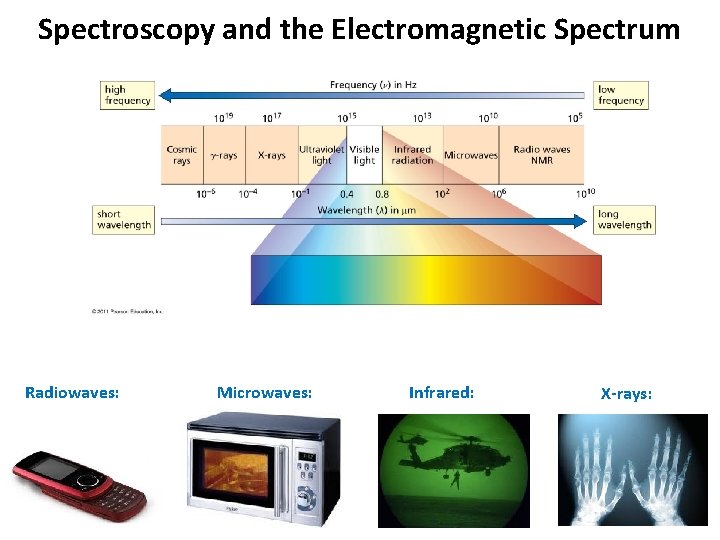
Spectroscopy and the Electromagnetic Spectrum Radiowaves: Microwaves: Infrared: X-rays:
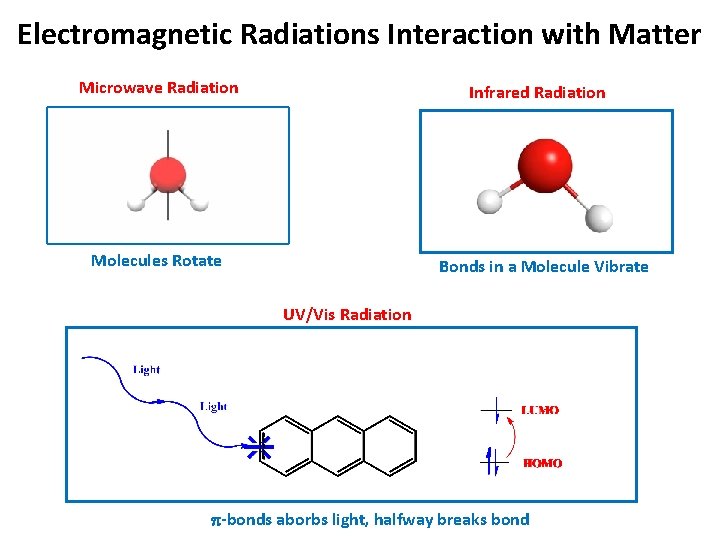
Electromagnetic Radiations Interaction with Matter Microwave Radiation Infrared Radiation Molecules Rotate Bonds in a Molecule Vibrate UV/Vis Radiation p-bonds aborbs light, halfway breaks bond
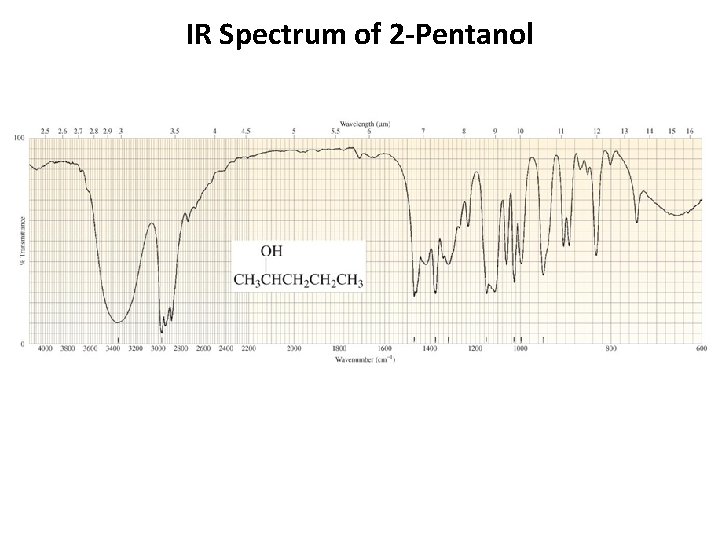
IR Spectrum of 2 -Pentanol
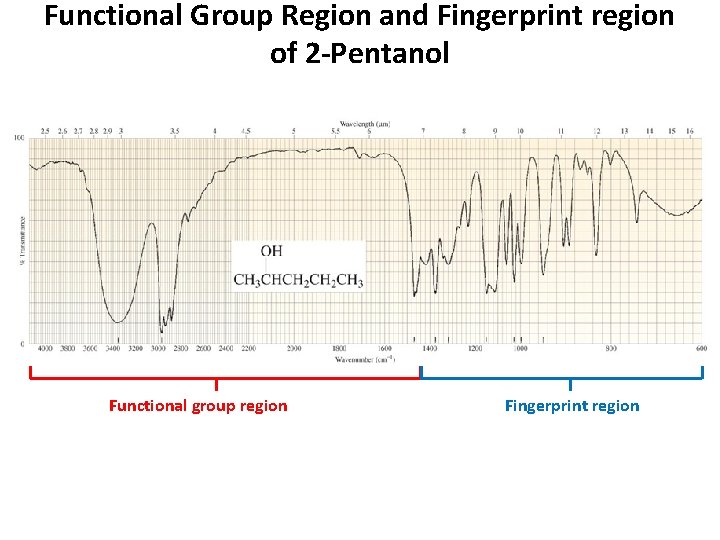
Functional Group Region and Fingerprint region of 2 -Pentanol Functional group region Fingerprint region
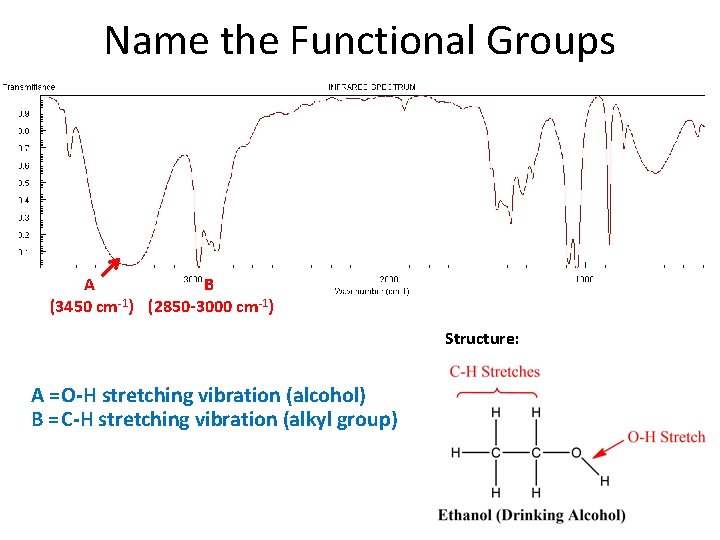
Name the Functional Groups A B (3450 cm-1) (2850 -3000 cm-1) Structure: A = O-H stretching vibration (alcohol) B = C-H stretching vibration (alkyl group)
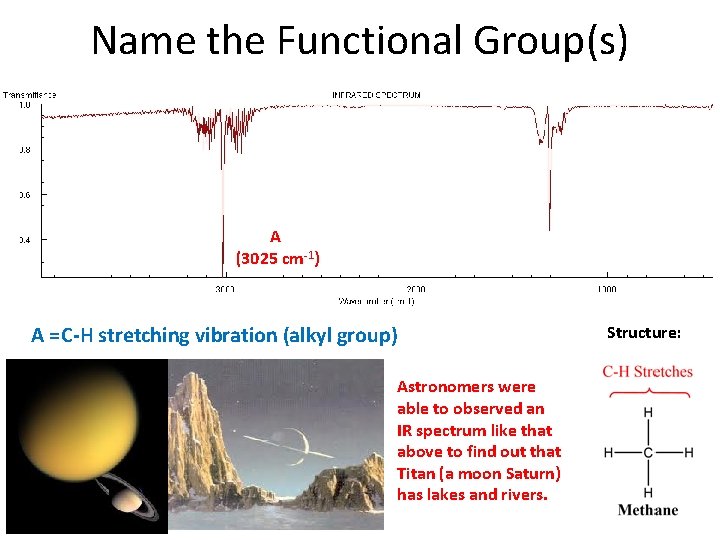
Name the Functional Group(s) A (3025 cm-1) A =C-H stretching vibration (alkyl group) Astronomers were able to observed an IR spectrum like that above to find out that Titan (a moon Saturn) has lakes and rivers. Structure:
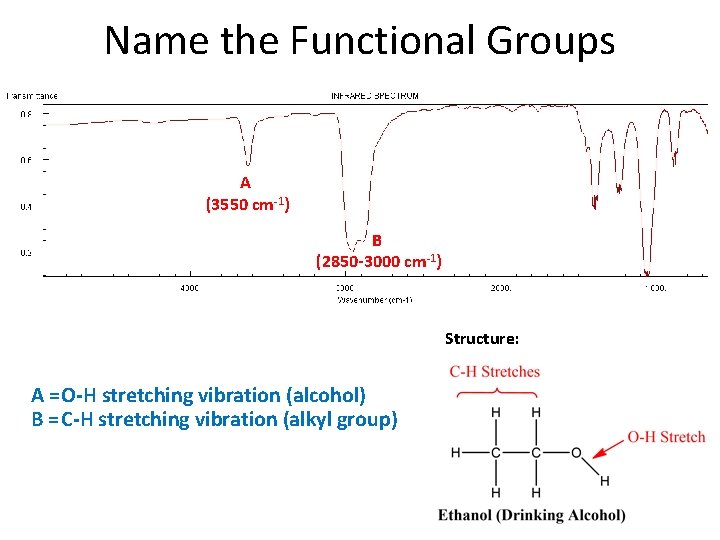
Name the Functional Groups A (3550 cm-1) B (2850 -3000 cm-1) Structure: A = O-H stretching vibration (alcohol) B = C-H stretching vibration (alkyl group)
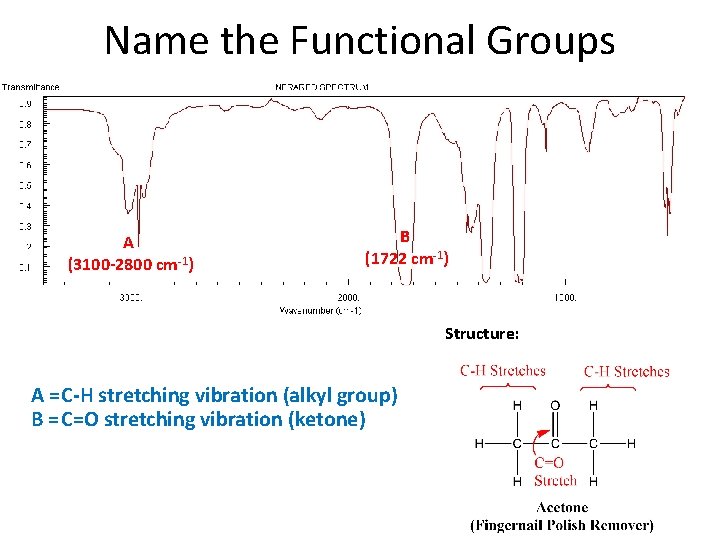
Name the Functional Groups A (3100 -2800 cm-1) B (1722 cm-1) Structure: A = C-H stretching vibration (alkyl group) B = C=O stretching vibration (ketone)
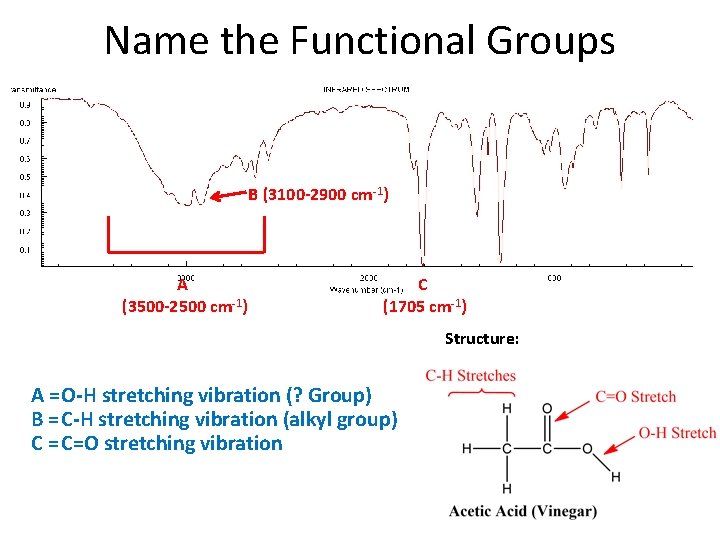
Name the Functional Groups B (3100 -2900 cm-1) A (3500 -2500 cm-1) C (1705 cm-1) Structure: A = O-H stretching vibration (? Group) B = C-H stretching vibration (alkyl group) C = C=O stretching vibration
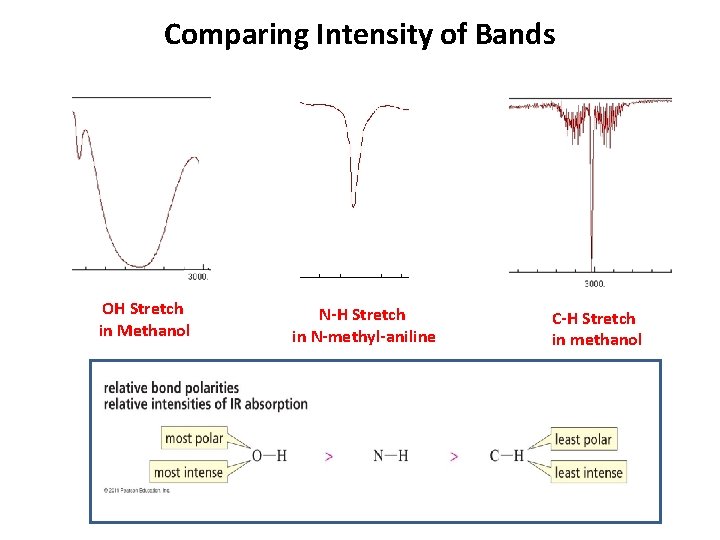
Comparing Intensity of Bands OH Stretch in Methanol N-H Stretch in N-methyl-aniline C-H Stretch in methanol
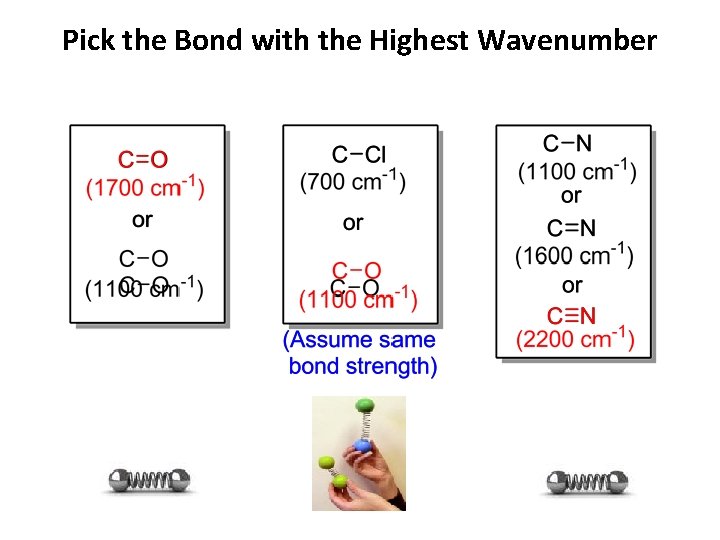
Pick the Bond with the Highest Wavenumber
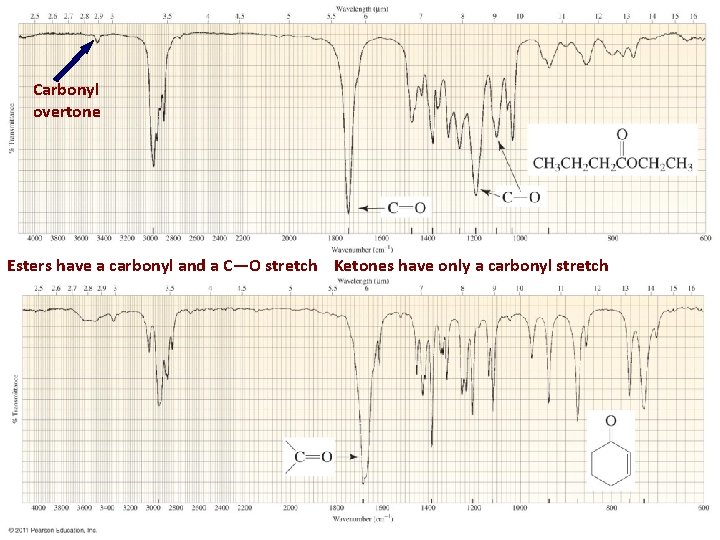
Carbonyl overtone Esters have a carbonyl and a C—O stretch Ketones have only a carbonyl stretch 24
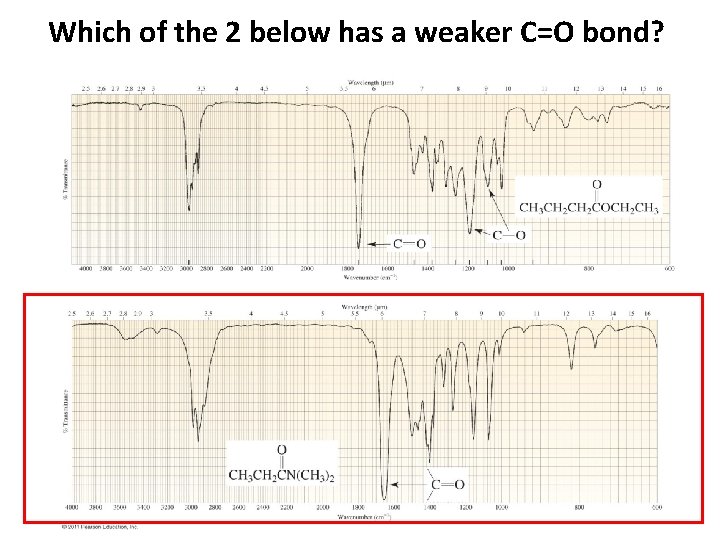
Which of the 2 below has a weaker C=O bond?
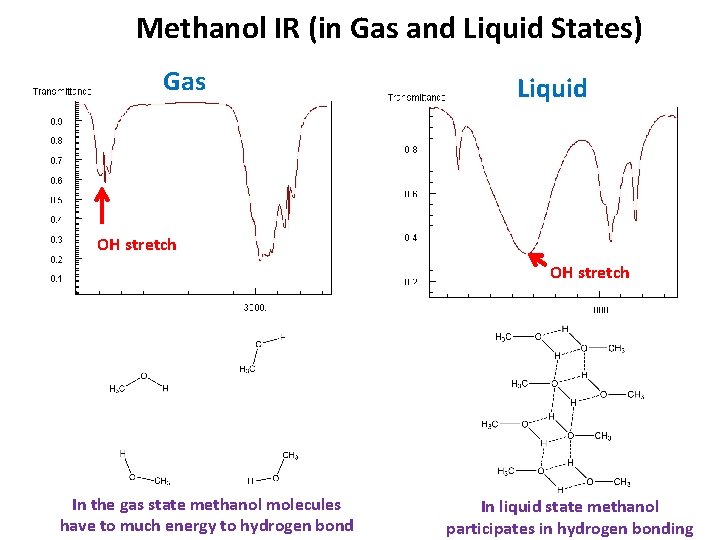
Methanol IR (in Gas and Liquid States) Gas Liquid OH stretch In the gas state methanol molecules have to much energy to hydrogen bond In liquid state methanol participates in hydrogen bonding
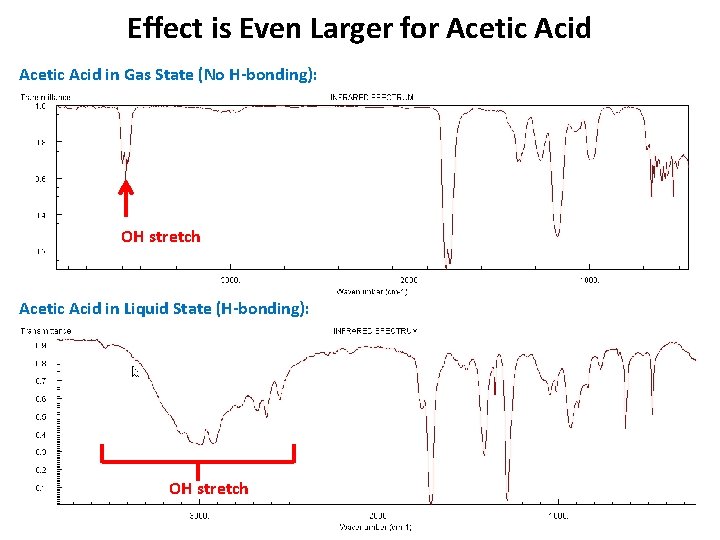
Effect is Even Larger for Acetic Acid in Gas State (No H-bonding): OH stretch Acetic Acid in Liquid State (H-bonding): OH stretch
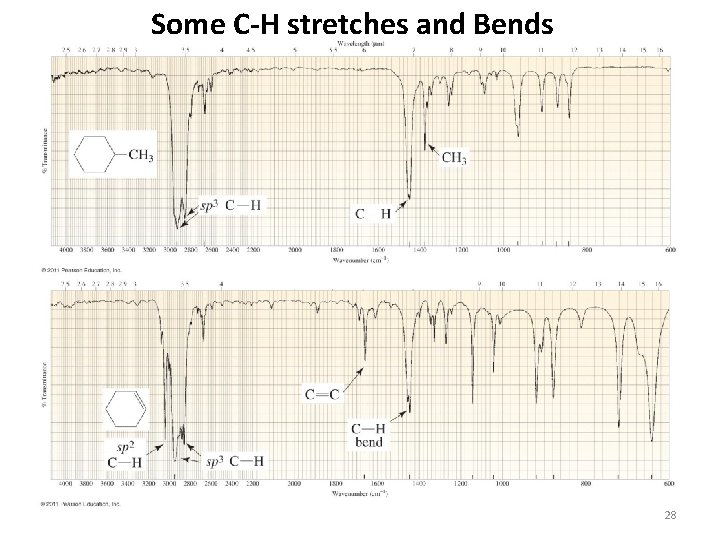
Some C-H stretches and Bends 28
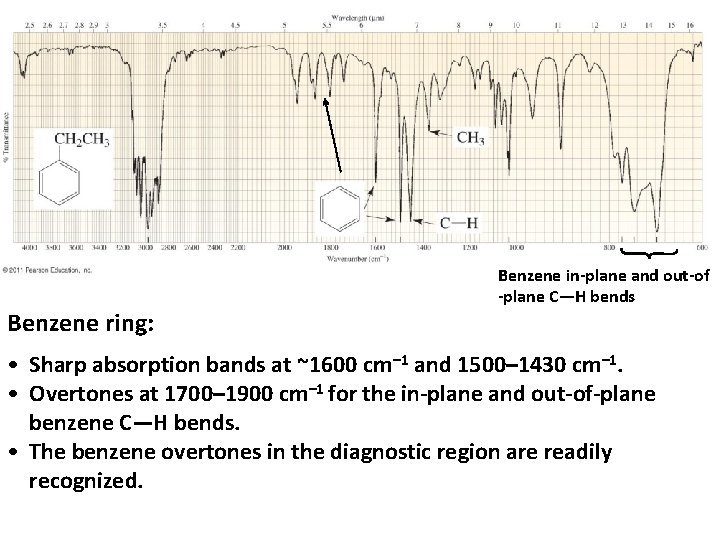
Benzene ring: Benzene in-plane and out-of -plane C—H bends • Sharp absorption bands at ~1600 cm– 1 and 1500– 1430 cm– 1. • Overtones at 1700– 1900 cm– 1 for the in-plane and out-of-plane benzene C—H bends. • The benzene overtones in the diagnostic region are readily recognized.
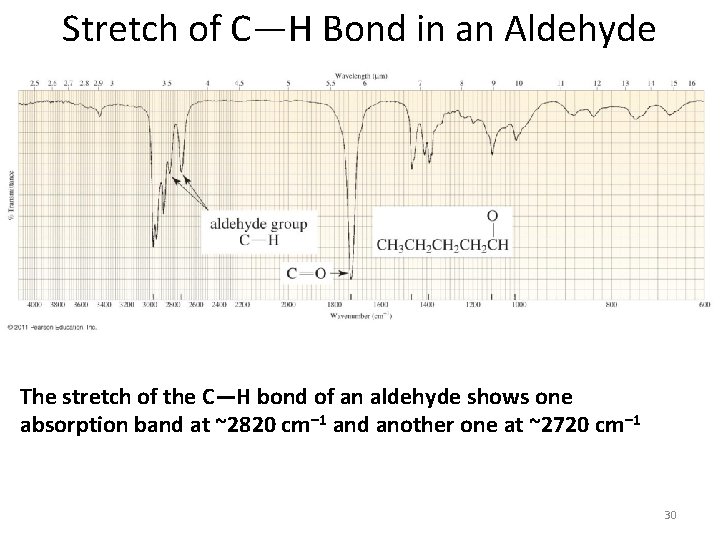
Stretch of C—H Bond in an Aldehyde The stretch of the C—H bond of an aldehyde shows one absorption band at ~2820 cm– 1 and another one at ~2720 cm– 1 30
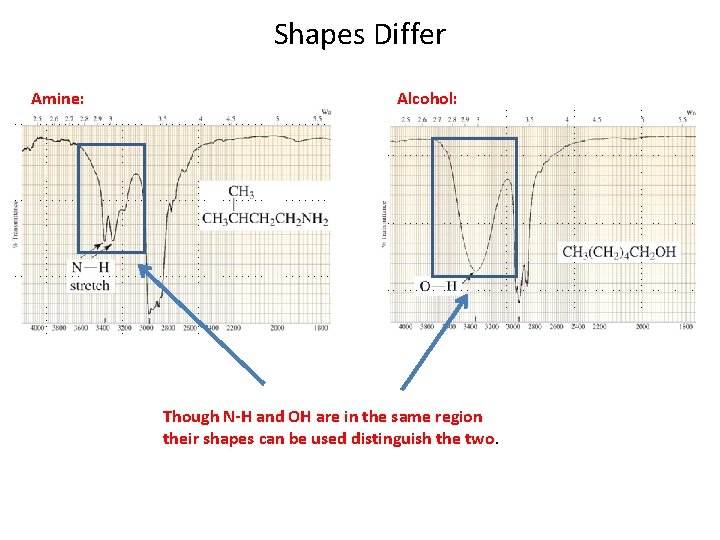
Shapes Differ Amine: Alcohol: Though N-H and OH are in the same region their shapes can be used distinguish the two.
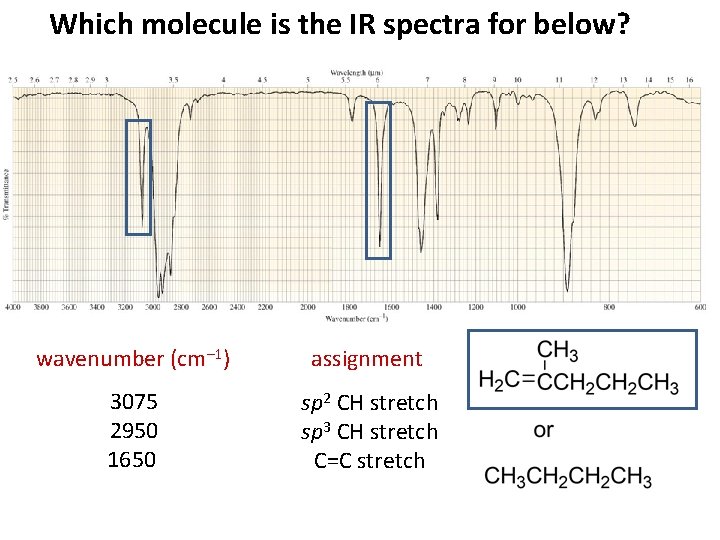
Which molecule is the IR spectra for below? wavenumber (cm– 1) assignment 3075 2950 1650 sp 2 CH stretch sp 3 CH stretch C=C stretch
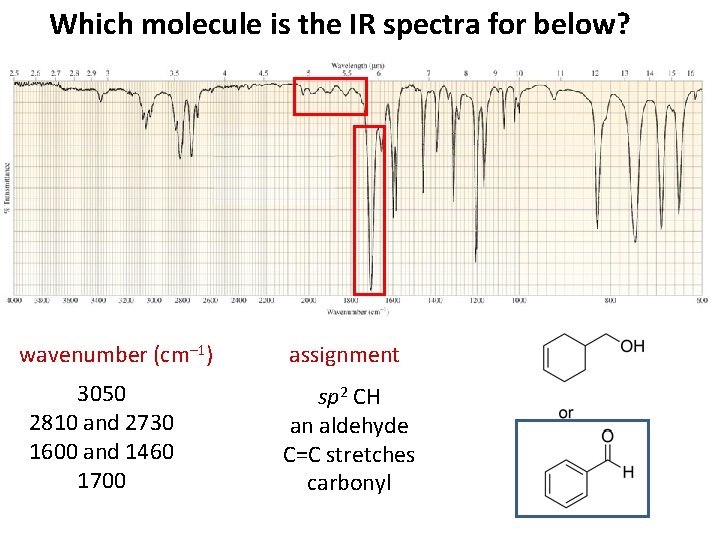
Which molecule is the IR spectra for below? wavenumber (cm– 1) 3050 2810 and 2730 1600 and 1460 1700 assignment sp 2 CH an aldehyde C=C stretches carbonyl
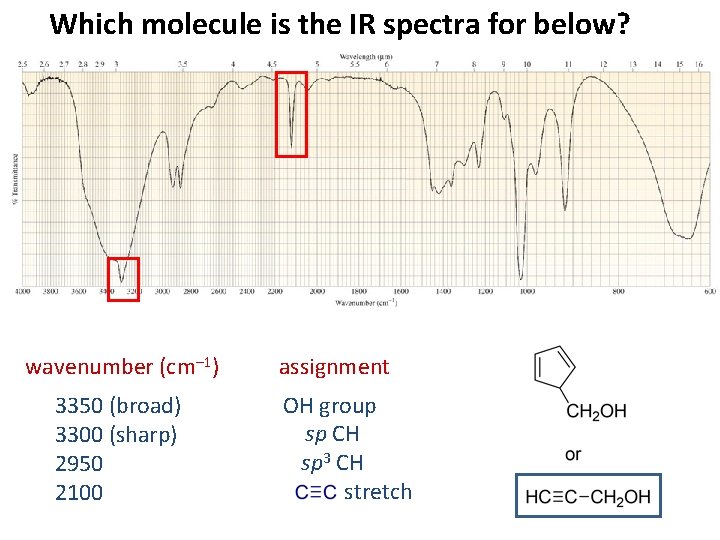
Which molecule is the IR spectra for below? wavenumber (cm– 1) 3350 (broad) 3300 (sharp) 2950 2100 assignment OH group sp CH sp 3 CH stretch
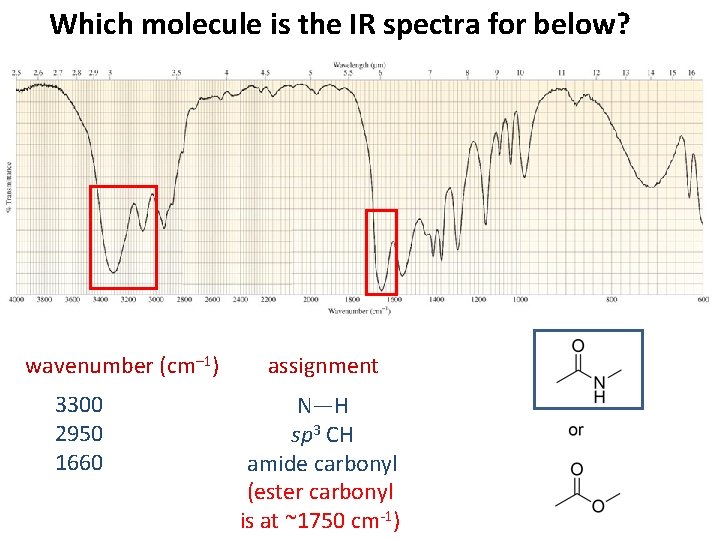
Which molecule is the IR spectra for below? wavenumber (cm– 1) 3300 2950 1660 assignment N—H sp 3 CH amide carbonyl (ester carbonyl is at ~1750 cm-1)
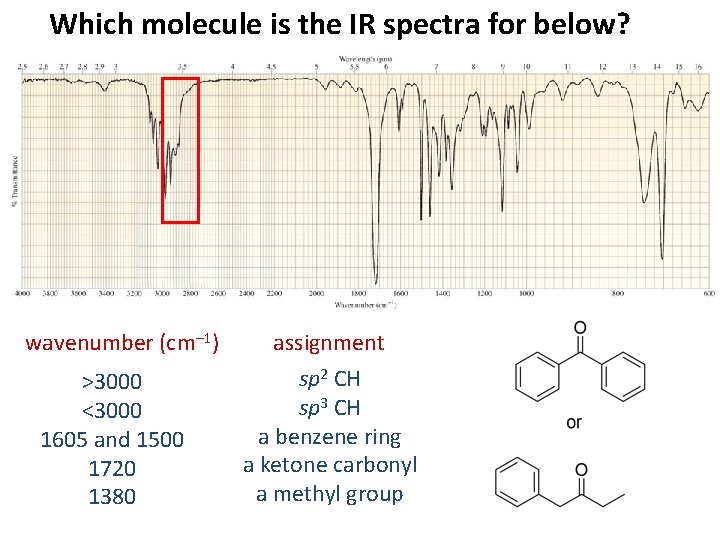
Which molecule is the IR spectra for below? wavenumber (cm– 1) >3000 <3000 1605 and 1500 1720 1380 assignment sp 2 CH sp 3 CH a benzene ring a ketone carbonyl a methyl group
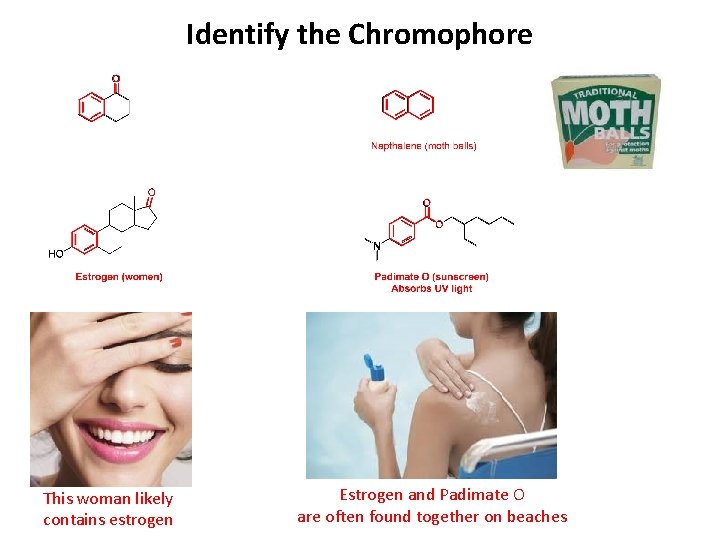
Identify the Chromophore This woman likely contains estrogen Estrogen and Padimate O are often found together on beaches
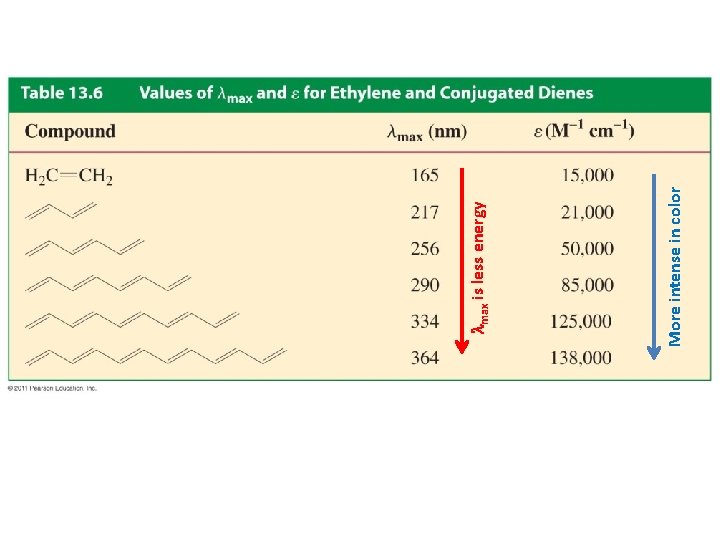
More intense in color lmax is less energy

Natural Colored Chromophores b-carotene powder Lycopene powder If a compound has enough conjugated double bonds, it will absorb visible light (lmax > 400 nm), and the compound will be colored b-carotene is the pigment responsible For the color of carrots, apricots and yams Lycopene colors Tomatoes, watermelon And pink grapefruit
- Slides: 39