Spectra Lab Spectra Lab Purpose We will observe
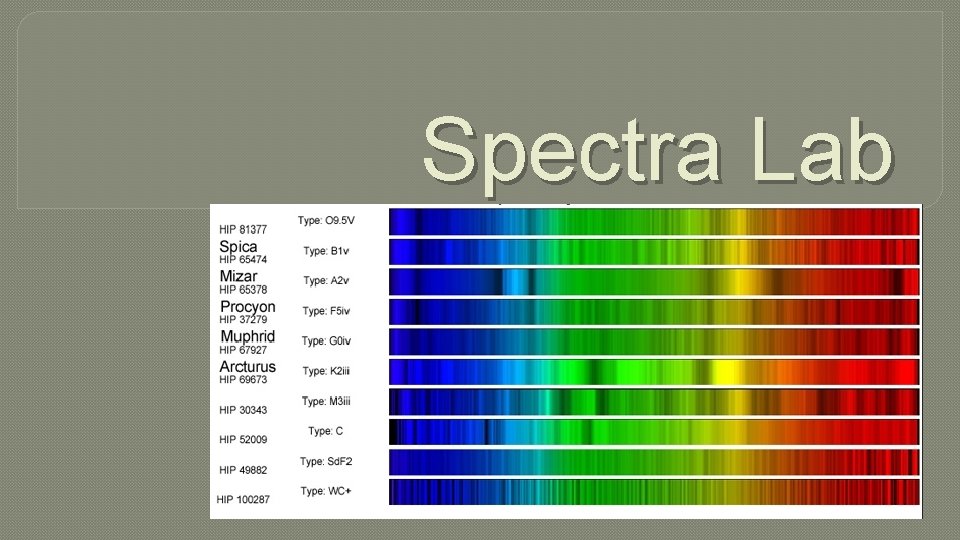
Spectra Lab

Spectra Lab Purpose: We will observe and draw the three different types of spectra. Materials: Spectroscopes Light sources Colored pencils/crayons

Research �Vocab: �spec·trum (ˈspektrəm/ noun)-1. a band of colors, as seen in a rainbow, produced by separation of the components of light by their different degrees of refraction according to wavelength. 2. the entire range of wavelengths of electromagnetic radiation. �in·can·des·cent (ˌinkənˈdes(ə)nt)light- emitting light as a result of being heated. �fluo·res·cent (ˌflo o(ə)ˈresənt, flôrˈesənt) light- absorbing light of short wavelength and emitting light of longer wavelength.
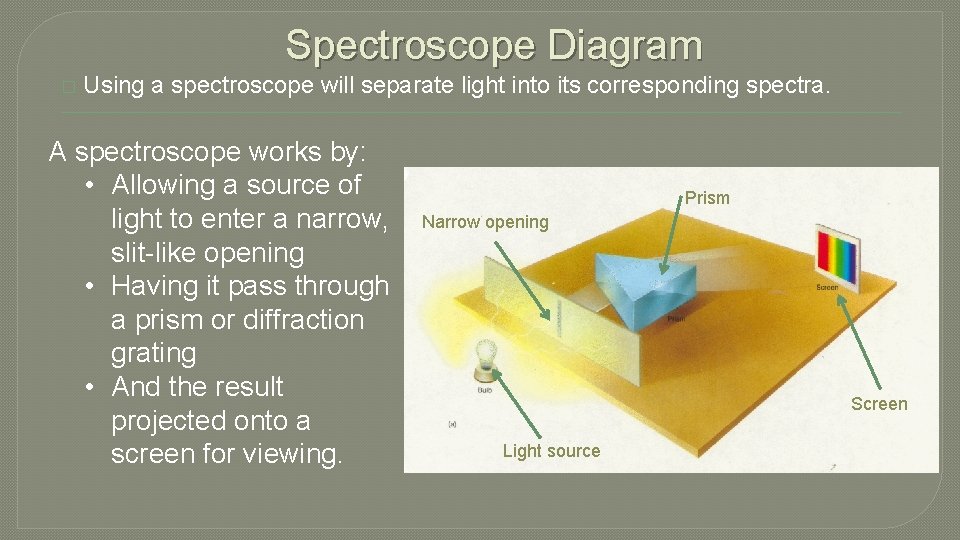
Spectroscope Diagram � Using a spectroscope will separate light into its corresponding spectra. A spectroscope works by: • Allowing a source of light to enter a narrow, slit-like opening • Having it pass through a prism or diffraction grating • And the result projected onto a screen for viewing. Prism Narrow opening Screen Light source

Research Kirchhoff's 3 Laws of Producing Spectra
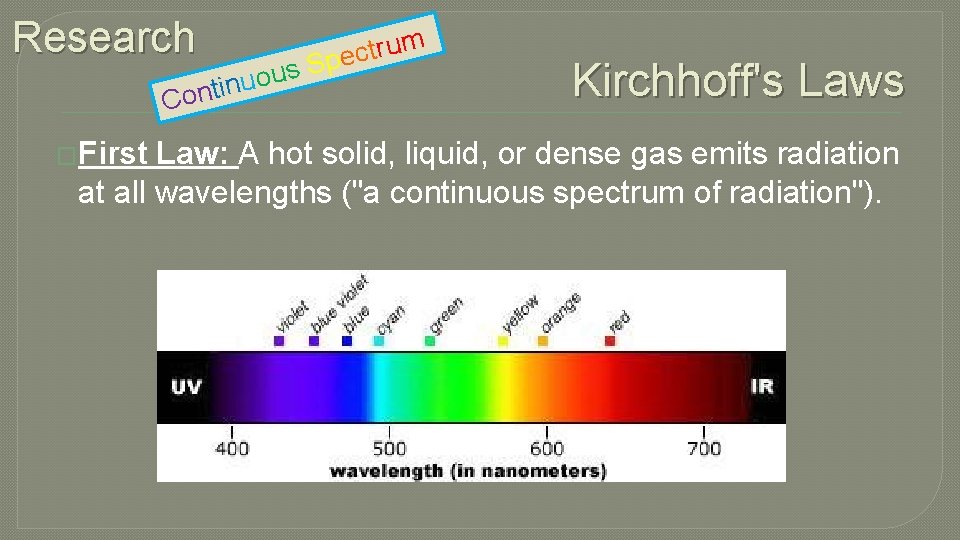
Research s ou u n i t on C �First m u r t c Spe Kirchhoff's Law: A hot solid, liquid, or dense gas emits radiation at all wavelengths ("a continuous spectrum of radiation").
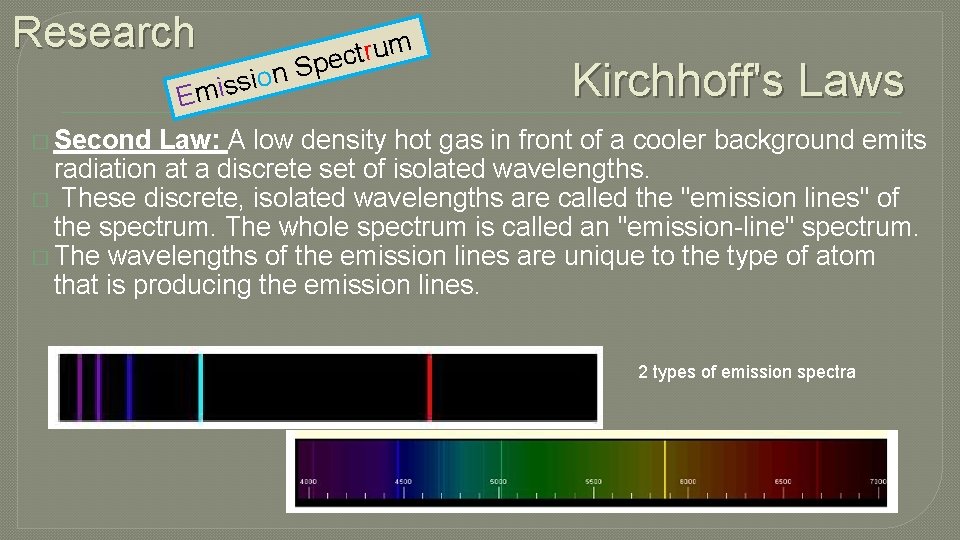
Research n o i s s E mi m u r t c Spe Kirchhoff's Laws � Second Law: A low density hot gas in front of a cooler background emits radiation at a discrete set of isolated wavelengths. � These discrete, isolated wavelengths are called the "emission lines" of the spectrum. The whole spectrum is called an "emission-line" spectrum. � The wavelengths of the emission lines are unique to the type of atom that is producing the emission lines. 2 types of emission spectra
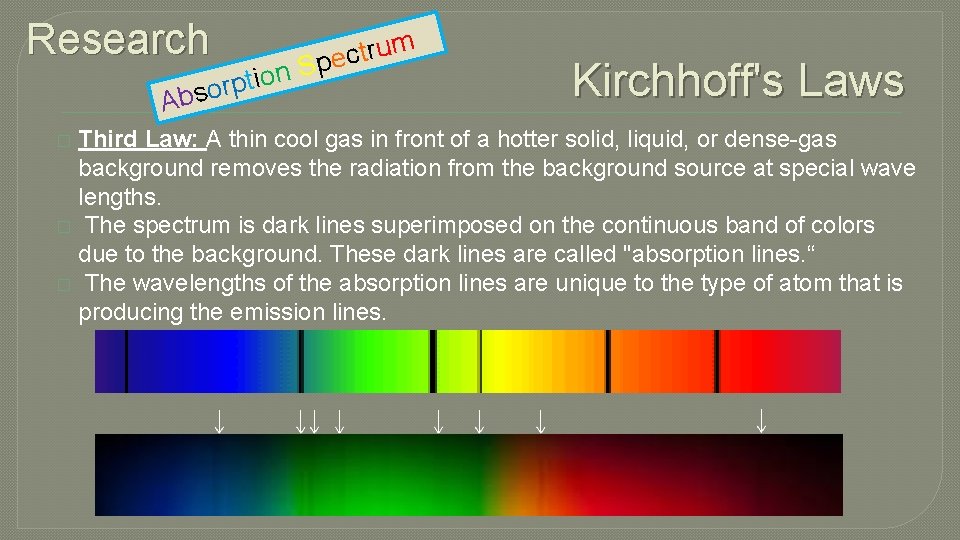
Research n o i t p bsor A m u r t c S pe Kirchhoff's Laws Third Law: A thin cool gas in front of a hotter solid, liquid, or dense-gas background removes the radiation from the background source at special wave lengths. � The spectrum is dark lines superimposed on the continuous band of colors due to the background. These dark lines are called "absorption lines. “ � The wavelengths of the absorption lines are unique to the type of atom that is producing the emission lines. �
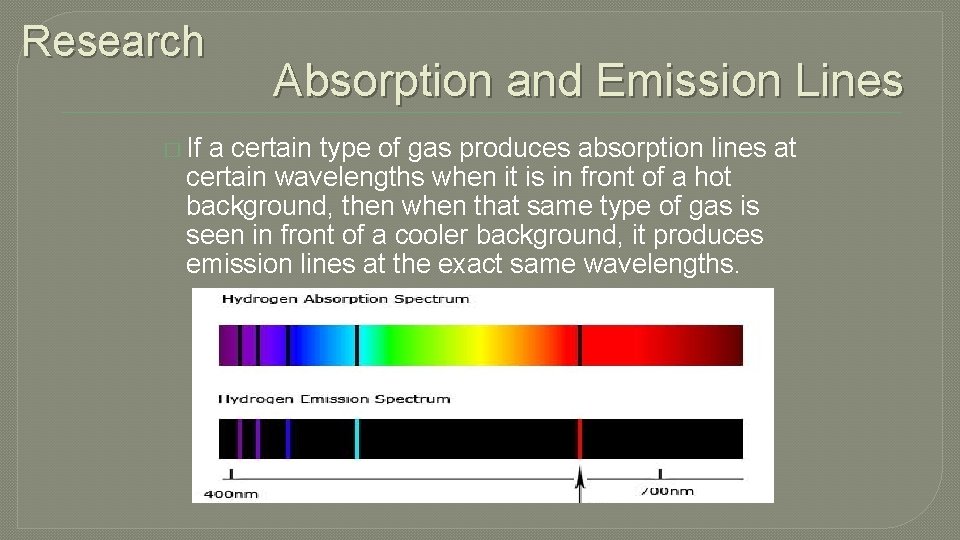
Research � If Absorption and Emission Lines a certain type of gas produces absorption lines at certain wavelengths when it is in front of a hot background, then when that same type of gas is seen in front of a cooler background, it produces emission lines at the exact same wavelengths.
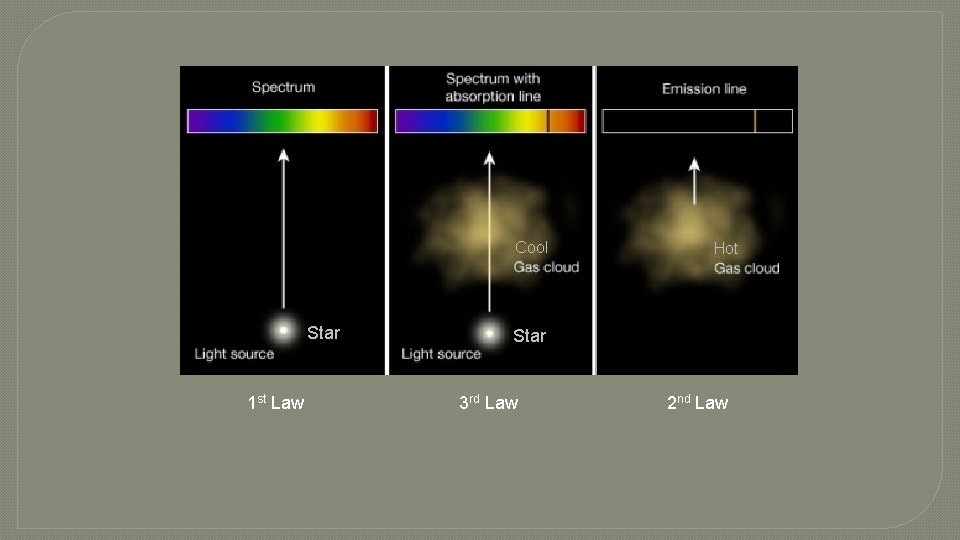
Cool Star 1 st Law Hot Star 3 rd Law 2 nd Law
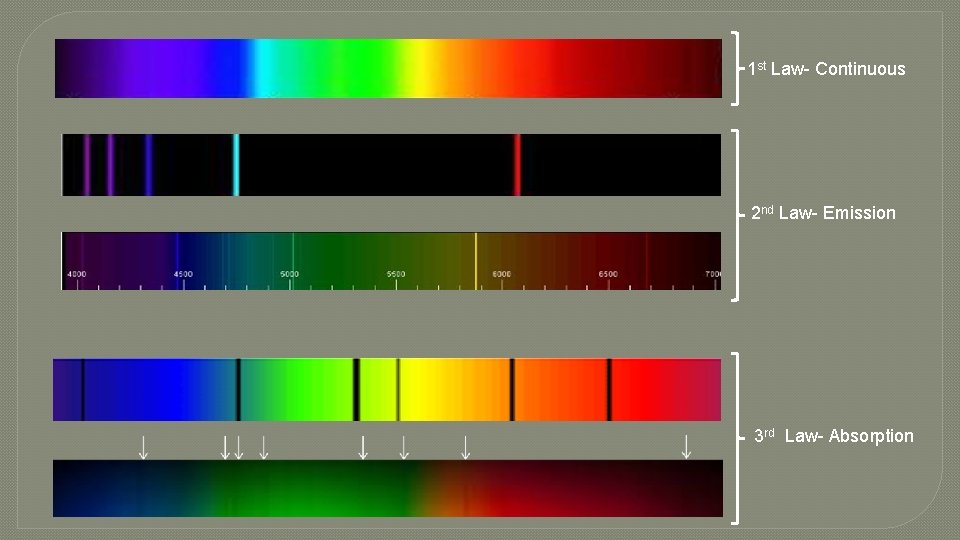
1 st Law- Continuous 2 nd Law- Emission 3 rd Law- Absorption

Procedure �Procedure: Using the spectroscopes, observe and draw the different spectra. Use colored pencils or crayons to color your spectra correctly. Label 2 things: • The type of spectrum it is (Continuous, Emission, Absorption) • Which of Kirschhoff’s laws is shown (1 st, 2 nd , or 3 rd Law)
- Slides: 12