SPECTACULAR REDOX REACTION BEHAVIOR 1 Thermite reaction 1assign
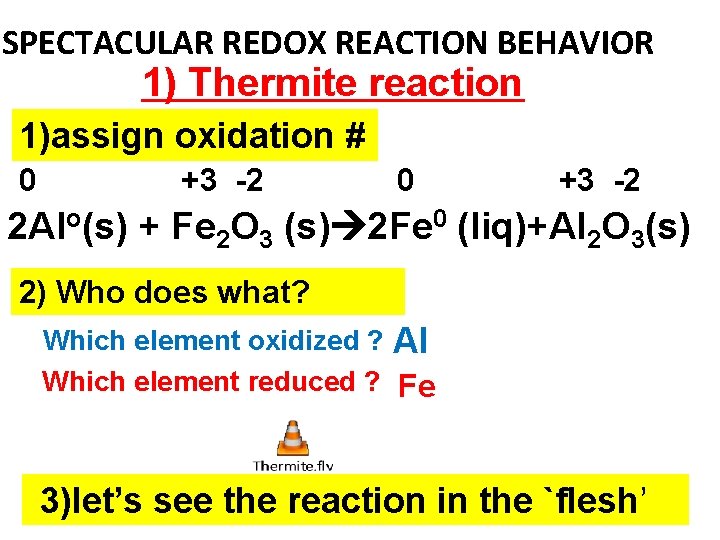
SPECTACULAR REDOX REACTION BEHAVIOR 1) Thermite reaction 1)assign oxidation # 0 +3 -2 2 Alo(s) + Fe 2 O 3 (s) 2 Fe 0 (liq)+Al 2 O 3(s) 2) Who does what? Which element oxidized ? Al Which element reduced ? Fe 3)let’s see the reaction in the `flesh’
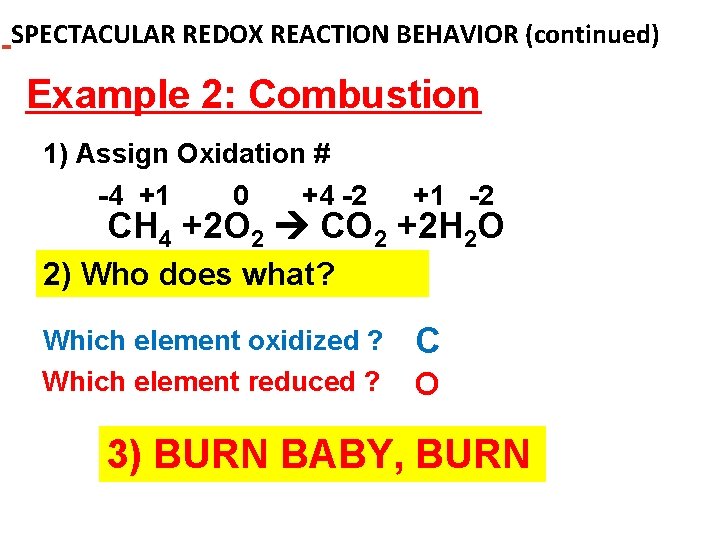
SPECTACULAR REDOX REACTION BEHAVIOR (continued) Example 2: Combustion 1) Assign Oxidation # -4 +1 0 +4 -2 +1 -2 CH 4 +2 O 2 CO 2 +2 H 2 O 2) Who does what? Which element oxidized ? Which element reduced ? C O 3) BURN BABY, BURN
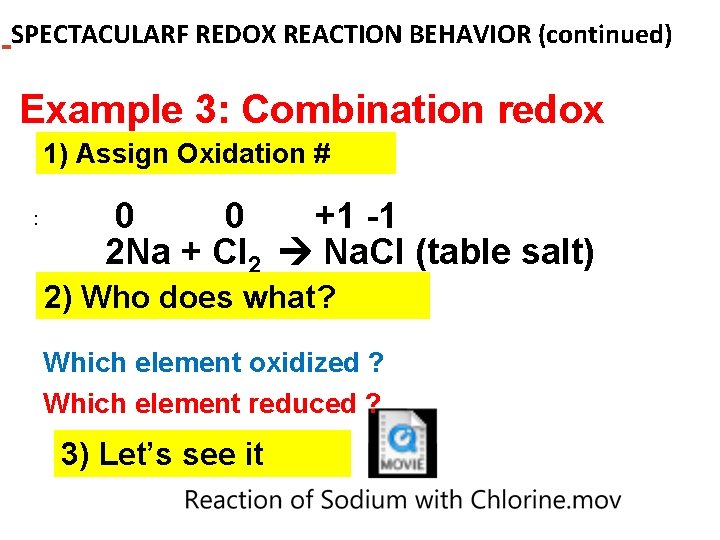
SPECTACULARF REDOX REACTION BEHAVIOR (continued) Example 3: Combination redox 1) Assign Oxidation # : 0 0 +1 -1 2 Na + Cl 2 Na. Cl (table salt) 2) Who does what? Which element oxidized ? Which element reduced ? 3) Let’s see it
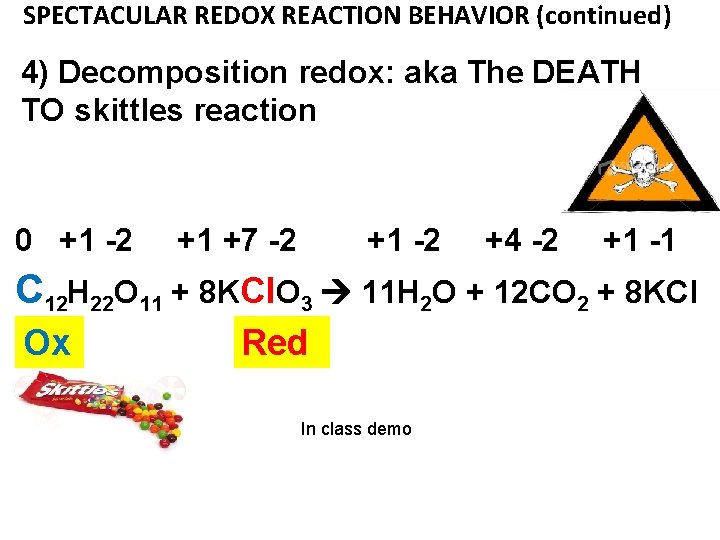
SPECTACULAR REDOX REACTION BEHAVIOR (continued) 4) Decomposition redox: aka The DEATH TO skittles reaction 0 +1 -2 +1 +7 -2 +1 -2 +4 -2 +1 -1 C 12 H 22 O 11 + 8 KCl. O 3 11 H 2 O + 12 CO 2 + 8 KCl Ox Red In class demo

One other unique and highly useful feature of redox reactions… The oxidation and reduction can be separated in space and you can still run the reaction as a `cell’ or ‘battery’
Characteristics of redox reactions • Heat and light common • Reactions are often spectacular (way better than acid base) Electronic balance altered (of course) Often accompanied by major decomposition or combination
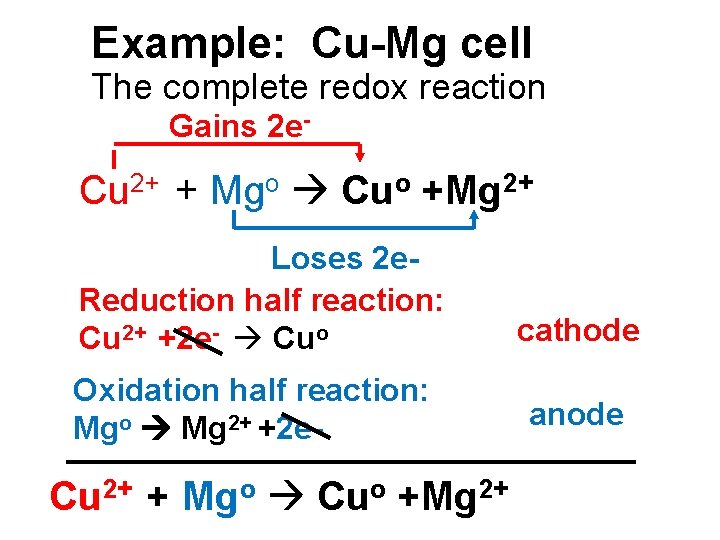
Example: Cu-Mg cell The complete redox reaction Gains 2 e- Cu 2+ + Mgo Cuo +Mg 2+ Loses 2 e. Reduction half reaction: Cu 2+ +2 e- Cuo Oxidation half reaction: Mgo Mg 2+ +2 e- Cu 2+ + Mgo Cuo +Mg 2+ cathode anode

Demos of Cu-Mg cell (battery) Potato clock
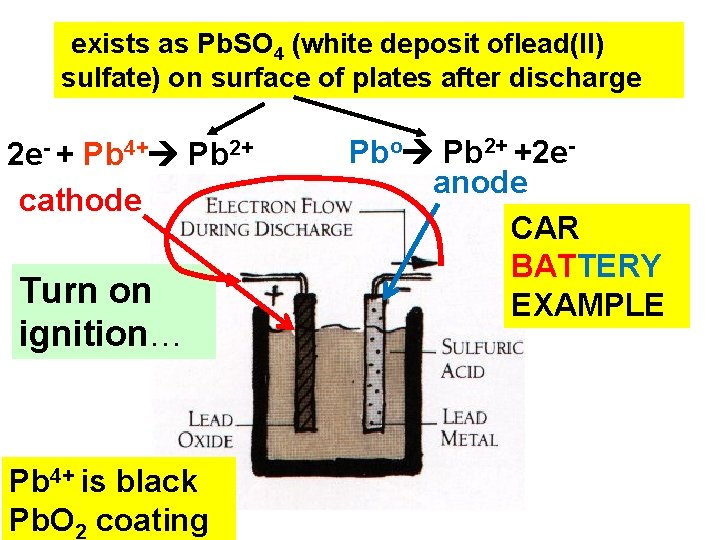
exists as Pb. SO 4 (white deposit oflead(II) sulfate) on surface of plates after discharge 2 e- + Pb 4+ Pb 2+ cathode Turn on ignition… Pb 4+ is black Pb. O 2 coating Pbo Pb 2+ +2 eanode CAR BATTERY EXAMPLE
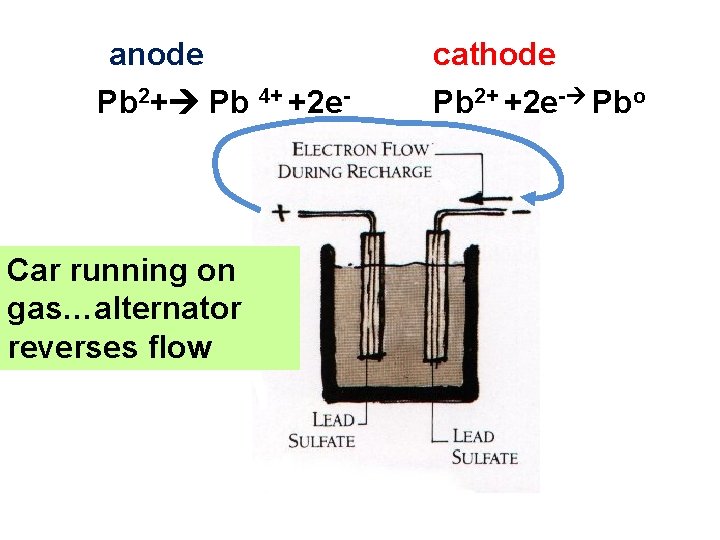
anode Pb 2+ Pb 4+ +2 e- Car running on gas…alternator reverses flow cathode Pb 2+ +2 e- Pbo
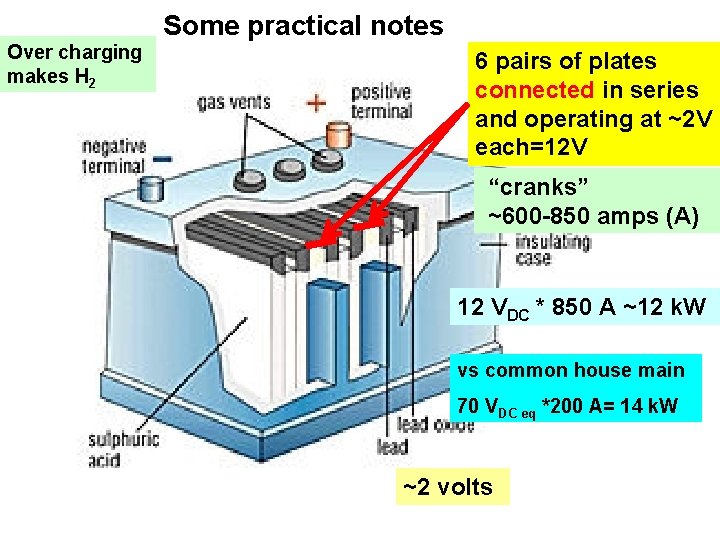
Some practical notes Over charging makes H 2 6 pairs of plates connected in series and operating at ~2 V each=12 V “cranks” ~600 -850 amps (A) 12 VDC * 850 A ~12 k. W vs common house main 70 VDC eq *200 A= 14 k. W ~2 volts

Exercise 9 a and 9 b… review of classical reaction ideas and language
- Slides: 12