SPECIMEN HANDLING AND COLLECTION Patient Identification Two or




















- Slides: 30

SPECIMEN HANDLING AND COLLECTION

Patient Identification Two or three items of identification should be used including: -name -medical record -date of birth -social security number -address if the patient is an outpatient

Types of Specimens 1 -Whole blood 2 -Serum. 3 -Plasma 4 -Urine 5 -Stool 6 -Saliva 7 -Other body fluids such as spinal, synovial, amniotic, pleural, pericardial and ascetic fluids 8 -Cells and various types of solid tissue

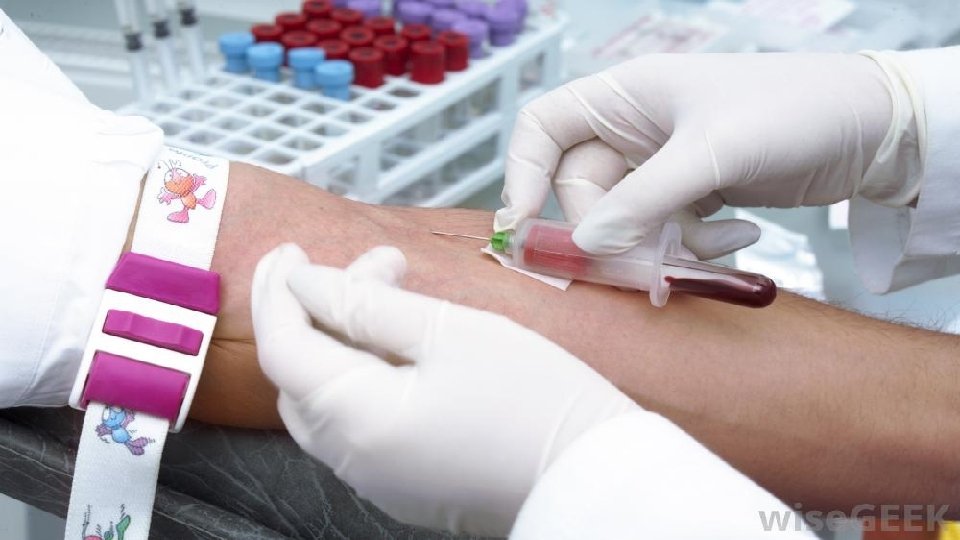
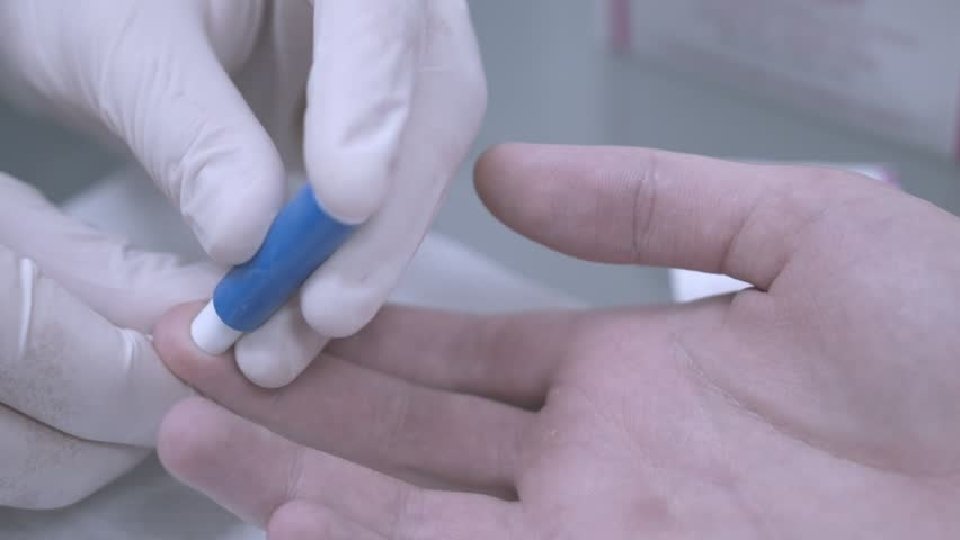
The process of collection of blood is known as phlebotomy 1. Venipuncture: - blood is collected through a needle inserted in the vein 2. Arterial puncture is used mainly for blood gas analyses 3. Capillary puncture: - blood is collected from a skin puncture made with a lancet or similar device.



venipuncture To obtain blood for a specimen To infuse fluids and blood To administer medication Diagnostic tests

criteria of a suitable vein Large enough to receive the shaft of the needle Visible and palpable Intact Use veins in uninjured arm Do NOT use veins if IV fluids are being administered Do NOT use veins that are thrombosed, tortuous or rolling

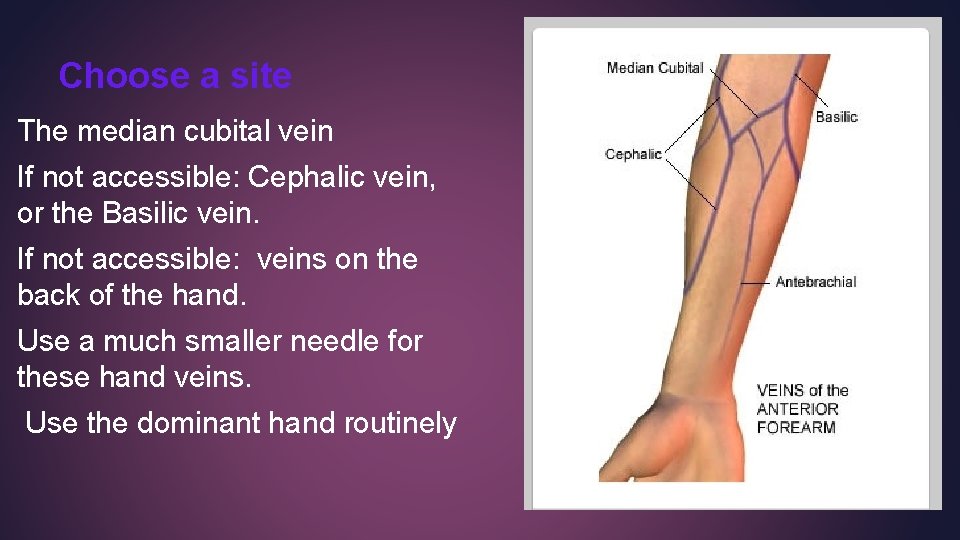
Choose a site The median cubital vein If not accessible: Cephalic vein, or the Basilic vein. If not accessible: veins on the back of the hand. Use a much smaller needle for these hand veins. Use the dominant hand routinely

Never draw from these areas Scarred, abraded, or inflamed skin Arms containing IV catheters Oedematous arms Occluded Veins Shunts If a woman has had a mastectomy, arm veins on that side of the body should not be used because the surgery may have caused lymphostasis (blockade of normal lymph node drainage), affecting the blood composition.

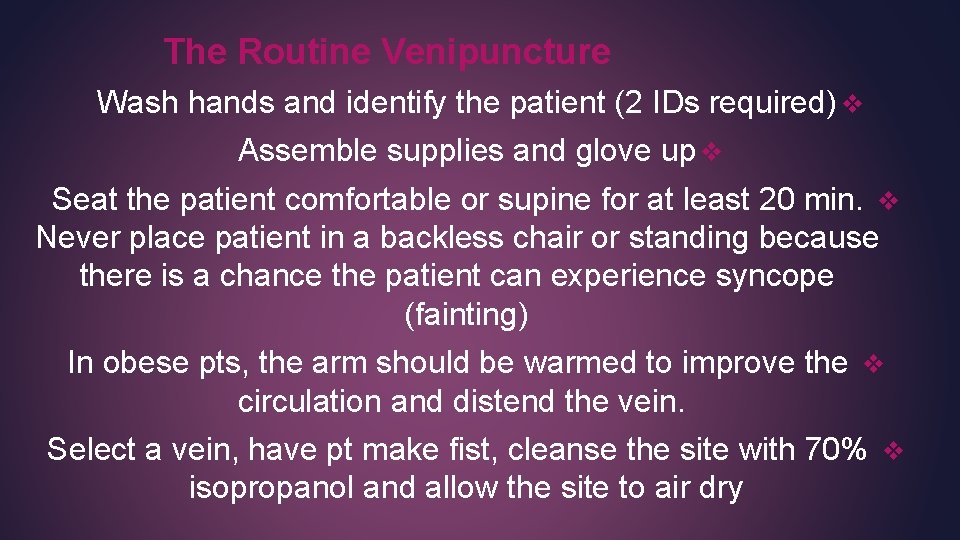
The Routine Venipuncture Wash hands and identify the patient (2 IDs required) v Assemble supplies and glove up v Seat the patient comfortable or supine for at least 20 min. v Never place patient in a backless chair or standing because there is a chance the patient can experience syncope (fainting) In obese pts, the arm should be warmed to improve the v circulation and distend the vein. Select a vein, have pt make fist, cleanse the site with 70% v isopropanol and allow the site to air dry

Apply tourniquet 2 -4 inches above site for no longer than 1 min v Anchor vein with thumb 1 -2 inches below puncture site and insert v needle, at 15 -degree angle with skin. Release and remove tourniquet as soon as blood is obtained v Patient’s hand should be relaxed and open v Place dry gauze lightly over puncture site (do not press down while v needle is in arm) Remove the needle and apply direct pressure to the puncture site using v the dry gauze.

Bandage the site when bleeding has stopped and remove after 15 min. v Properly dispose of puncture equipment and biohazardous waste v Label the tubes (name, ID, date, time) v Send tubes for analysis v Release patient from diet restrictions (if any). v

Order of Draw for Multiple Blood Specimens This is applicable for both glass and plastic collection tubes when drawing multiple specimens for clinical laboratory testing during a single venipuncture. 1) Blood culture tube 2) Coagulation tube (e. g. , blue closure) 3) Serum tube with or without clot activator, with or without gel (e. g. , red closure) 4) Heparin tube with or without gel plasma separator (e. g. , green closure) 5) EDTA tube with or without gel separator (e. g. , lavender closure, pearl closure) 6) Glycolytic inhibitor (e. g. , gray closure).

Complications of venipuncture. 1) Hematoma: a) Needle has gone through the vein b) Bevel is only partially in the vein c) Insufficient pressure on a puncture site after needle is removed 2) Phlebitis: May result from repeated puncture of vein and/or improper technique 3) Septicemia: Results from using contaminated equipment 4) Trauma: Usually caused by probing with the needle

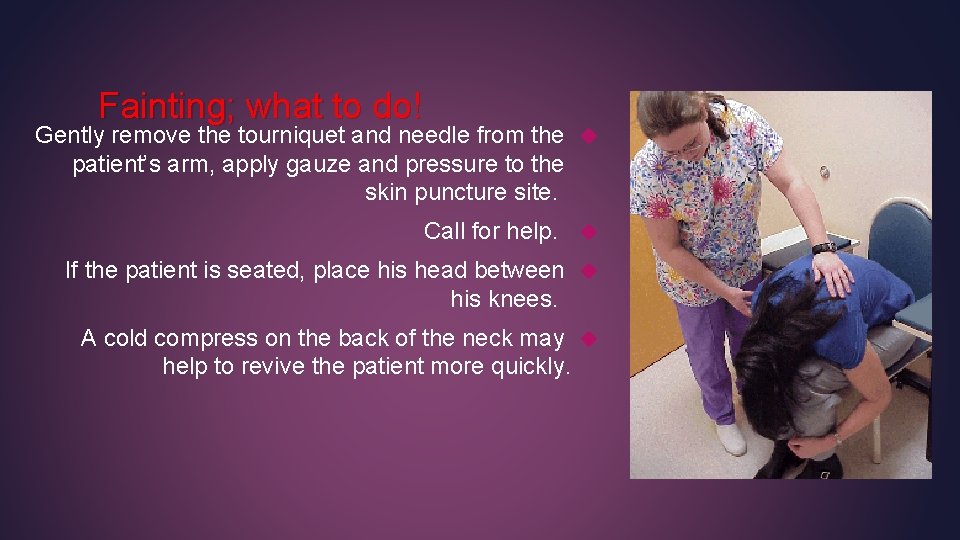
Fainting; what to do! Gently remove the tourniquet and needle from the patient’s arm, apply gauze and pressure to the skin puncture site. Call for help. If the patient is seated, place his head between his knees. A cold compress on the back of the neck may help to revive the patient more quickly.

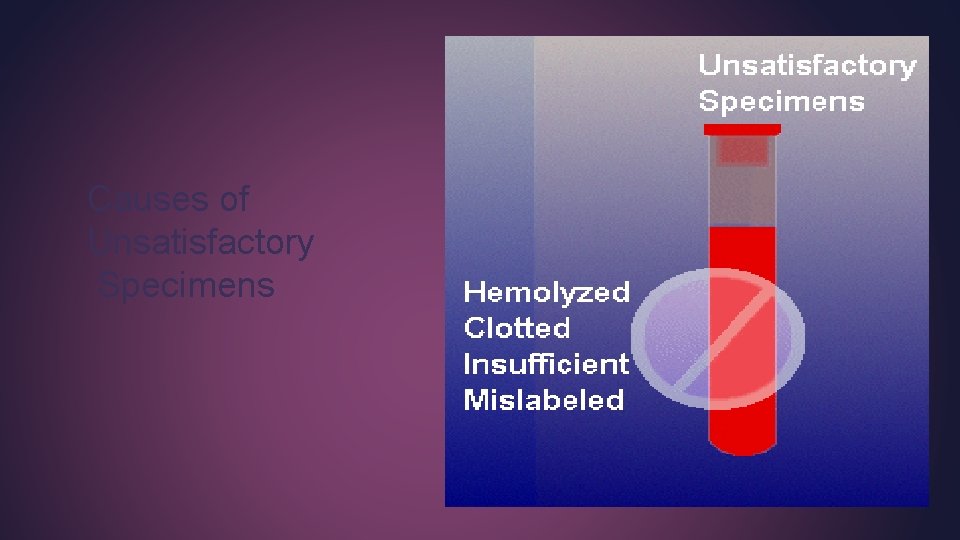
Causes of Unsatisfactory Specimens

Hemolysis Causes of Hemolysis Using a too small needle for a relatively bigger vein Pulling a syringe plunger too rapidly Expelling blood vigorously into a tube, Using a wet tube, Shaking a tube of blood too hard.

Effects Of Hemolysis: Hemolysis can cause falsely increased potassium, magnesium, proteins, iron, ammonia levels, and many enzymes including aldolase and LDH (constituents of RBCs). Increases inorganic phosphorus due to hydrolysis of organic esters released from the cells. Serum protein electrophoresis may show an additional band due to haemoglobin. Interferes with many colorimetric assays. The degree of interference depends on the method used.

Clots within the blood specimen, even if not visible to the naked eye, will yield inaccurate results. Causes of Clots: q Inadequate mixing of blood anticoagulant q Delay in expelling blood within a syringe into a collection tube

Insufficient volume short draws will result in an incorrect ratio of blood to anticoagulant and yield incorrect test results. Short draws can be caused by: 1. A vein collapsing during phlebotomy. 2. The needle coming out of the vein before the collection tube is full. 3. Loss of collection tube vacuum before the tube is full.

Labeling Errors Labeling errors are the most common cause of incorrect laboratory results. Contamination of the samples: 1 - Swabbing with ethanol prior to venepuncture can cause elevated blood alcohol level while swabbing with isopropanol can cause low blood alcohol level. 2 - Air bubbles in the syringes for ABG affect the p. H, p. O 2 & p. CO 2

Prolonged stasis: Prolonged stasis increases the concentration of protein and protein bound constituents such as Ca, bilirubin, cholesterol , TG, thyroxin, cortisol and other bound hormones or drugs. It can lead to local hypoxia and leakage of RBCs contents like hemolysis.

Supine vs. sitting or standing The following may decrease by 5 -15% in the supine patient: Total protein Albumin Lipids Iron Calcium Enzymes Ig Thyroxine

Anticoagulants and Preservatives for Blood A number of anticoagulants are available, including heparin, EDTA, sodium fluoride, citrate, acid citrate dextrose and oxalate

Heparin: . It is available as sodium, potassium, lithium, and ammonium salts, all of which can adequately prevent coagulation. This anticoagulant accelerates the action of antithrombin III, which neutralizes thrombin and thus prevents the formation of fibrin from fibrinogen. Citrate: Sodium citrate solution, at a concentration of 34 to 38 g/L in a ratio of 1 part to 9 parts of blood, is widely used for coagulation studies. Oxalates: Sodium, potassium, ammonium, and lithium oxalates inhibit blood coagulation by forming rather insoluble complexes with calcium ions. Ethylenediaminetetraacetic Acid (EDTA): EDTA is a chelating agent of divalent cations such as Ca +² and Mg+² that is particularly useful for: 1 -hematological examinations including transfusion medicine applications 2 -measurement of intracellular drugs such as cyclosporine or tacrolimus 3 -Hb. A 1 c analysis 4 -isolation of genomic DNA 5 -qualitative and quantitative virus determination by molecular techniques because it preserves the cellular component of blood . Sodium Fluoride: preservative for blood glucose and lactate. It inhibits the enzyme system involved in glycolysis, so it inhibits glycolysis and preserve glucose in the specimen for at least 24 hours

Urine: A clean, early morning, fasting specimen is usually the most concentrated specimen and this is preferred for microscopic examinations and for the detection of abnormal amount of constituents, such as proteins. Catheter specimens are used for microbiologic examination in critically ill patients. Stool Small aliquots of feces are frequently analyzed routinely known as routine stool analysis or to detect the presence of “hidden blood”, so- called occult blood.

Handling of Specimens For Analysis Steps that are important for obtaining a valid specimen for analysis include: 1 - Identification 2 - Preservation 3 - Separation and storage 4 - Transport

Specimen Identification: Bar code system is used in many laboratories to facilitate labelling, sample tracking and bar code readers are used in instruments for proper analysis Separation and Storage of Specimens Plasma or serum should be separated from cells as soon as possible and certainly within 2 hours. Premature separation of serum, however, may permit continued formation of fibrin, which can clog sampling devices in testing equipment. Transport of Specimens For some tests, specimens must be kept at 4˚C degradation of sample components such as ammonia, hormones For transfer of these specimens to the laboratory, the specimen container should be placed on ice. Specimens for bilirubin need to be protected from light to prevent photo degradation.

Thank you GOOD LUCK