Specifying Material Streams In the previous section you








- Slides: 26


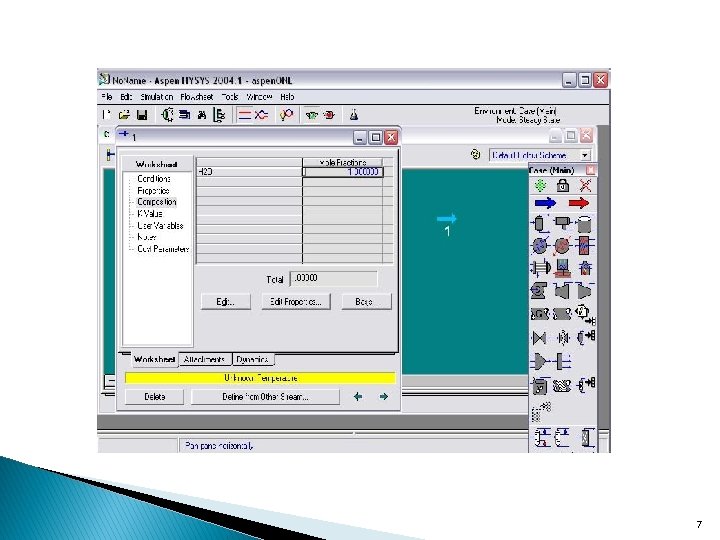
Specifying Material Streams In the previous section you specified the stream conditions in the Workbook property view. Next you will input the composition information in the Stream property view. The PFD becomes visible and displays a light blue arrow on it, labeled Feed 1. That arrow is the stream Feed 1 that you just created. 2

Alternative Methods for Defining Streams In addition to the method you just learned, there are several alternative ways to define streams. � Access the Object Palette by pressing F 4. Then Click the Material Stream icon on the Object Palette, then click on the Add Object icon. � Press F 11. � From the Flowsheet menu, select Add Stream. Each of the above three methods creates a new stream and access the property view of the new stream. 3

4

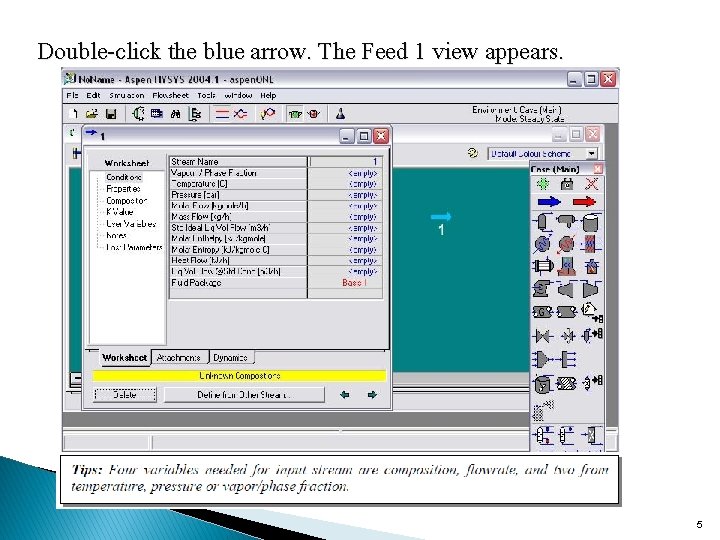
Double-click the blue arrow. The Feed 1 view appears. 5

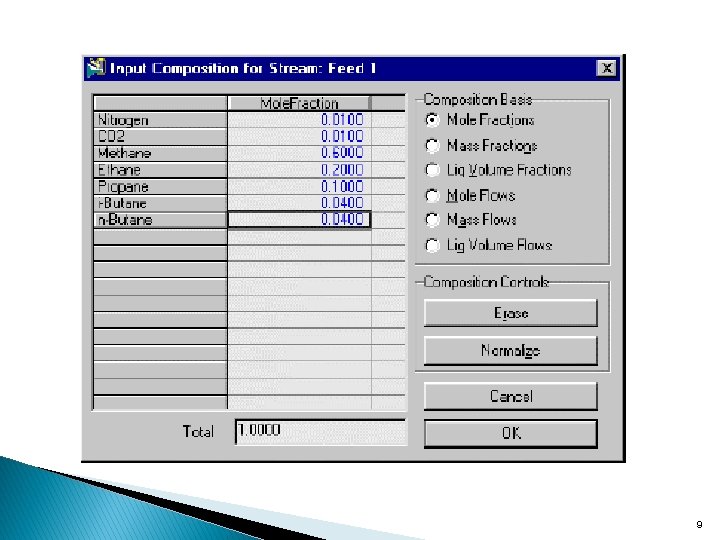
Specifying The Compositions Click on the Composition page. By default, the components are listed by Mole Fractions. When you have entered the fraction of each component the total at the bottom of the property view will equal 1. 0000. 6

7

The compositions currently appear in Mass Fraction. To change this, click the Basis button, then select the appropriate radio button in the Composition Basis group of the property view that appears. 8

9

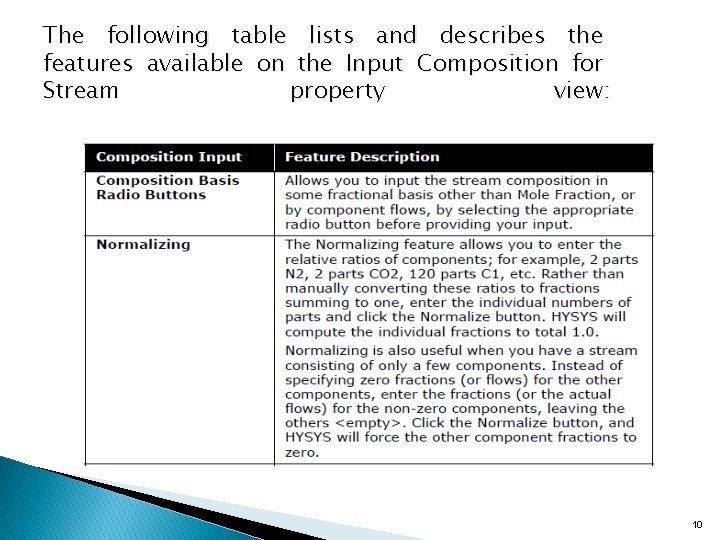
The following table lists and describes the features available on the Input Composition for Stream property view: 10

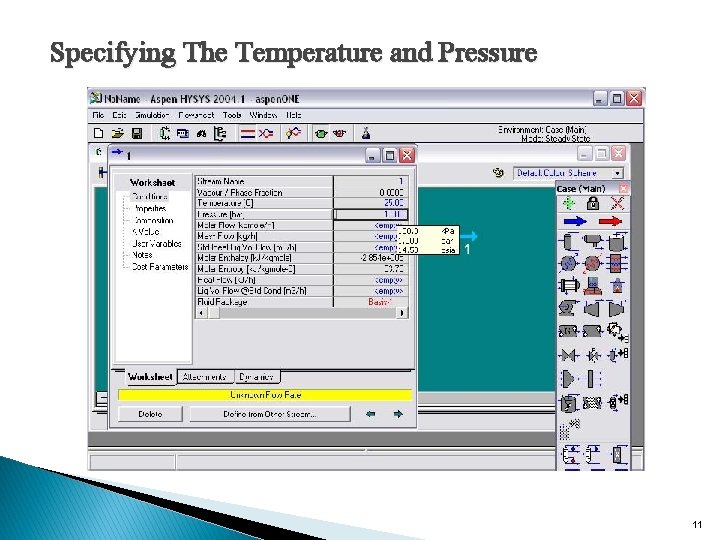
Specifying The Temperature and Pressure 11

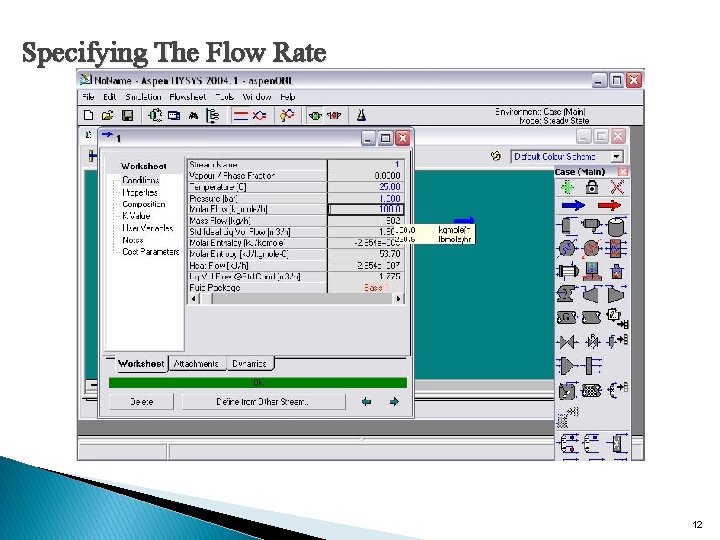
Specifying The Flow Rate 12

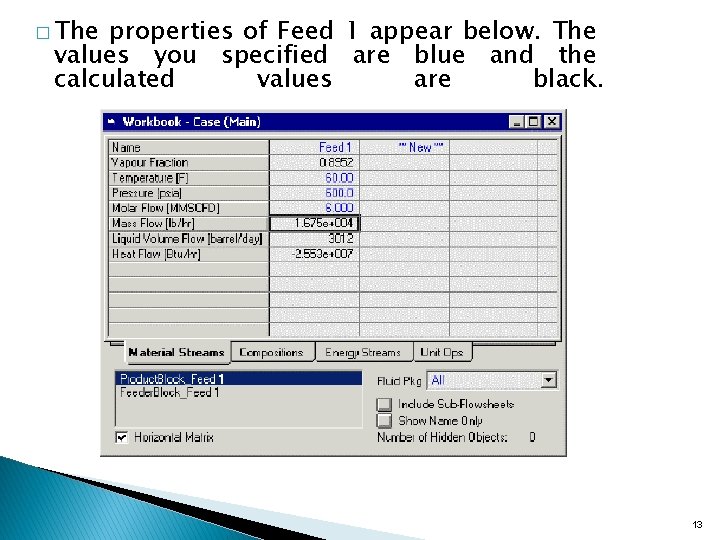
� The properties of Feed 1 appear below. The values you specified are blue and the calculated values are black. 13

Part 2 Equations of State The ideal gas equation of state , which relates the pressure, temperature, and specific volume, is a familiar equation: The term p is the absolute pressure , V is the volume, n is the number of moles , R is the gas constant , and T is the absolute temperature. This equation is quite adequate when the pressure is low (such as one atmosphere). However, many chemical processes take place at very high pressure. Under these condition , the ideal gas equation of state may not be valid representation of reality. 14

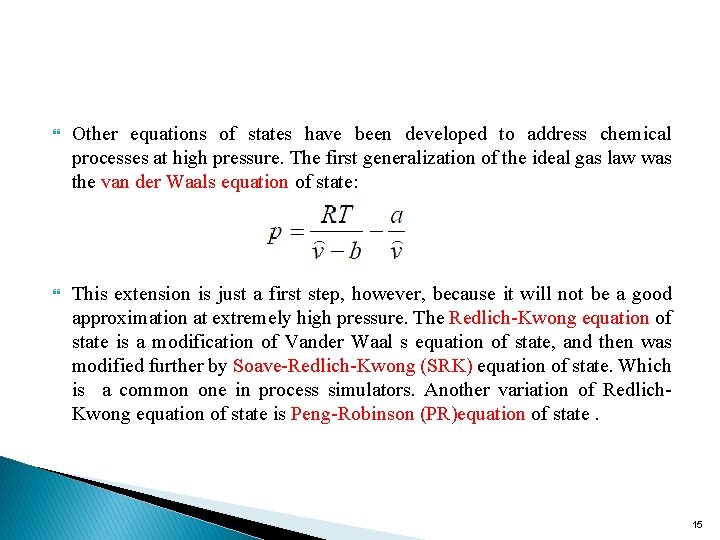
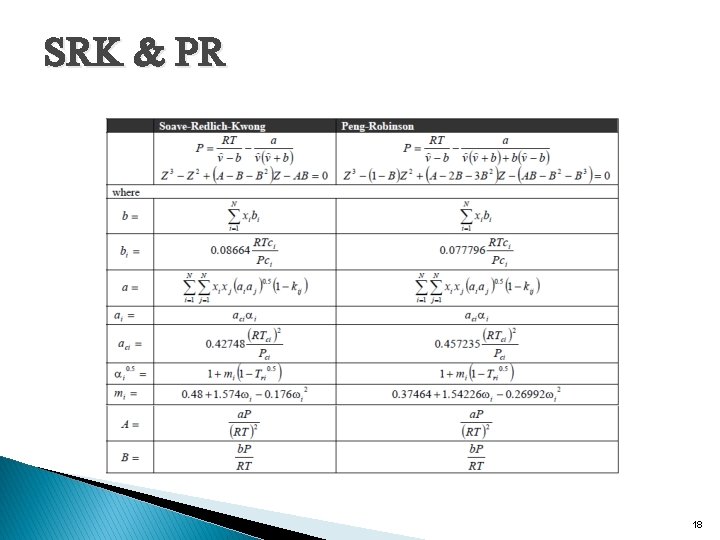
Other equations of states have been developed to address chemical processes at high pressure. The first generalization of the ideal gas law was the van der Waals equation of state: This extension is just a first step, however, because it will not be a good approximation at extremely high pressure. The Redlich-Kwong equation of state is a modification of Vander Waal s equation of state, and then was modified further by Soave-Redlich-Kwong (SRK) equation of state. Which is a common one in process simulators. Another variation of Redlich. Kwong equation of state is Peng-Robinson (PR)equation of state. 15

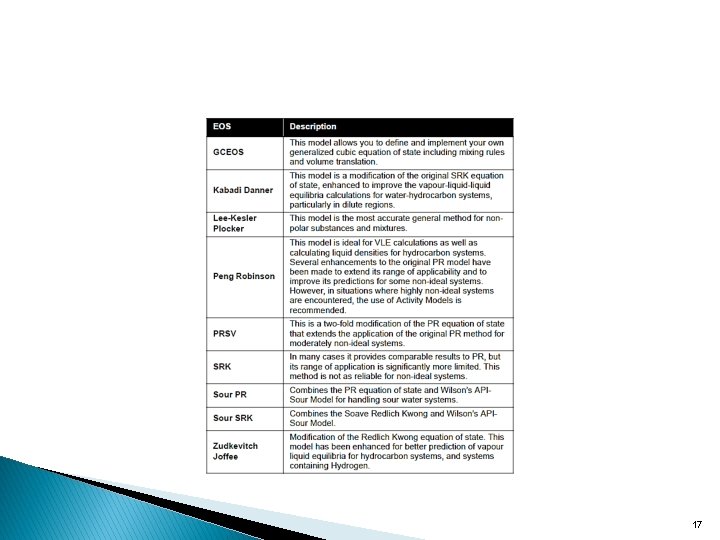
For oil, gas and petrochemical applications, the Peng Robinson Equation of State is generally the recommended property package. Hyprotech’s enhancements to this equation of state enable it to be accurate for a variety of systems over a wide range of conditions. It rigorously solves most single phase, two phase and three-phase systems with a high degree of efficiency and reliability. All equation of state methods and their specific applications are described below: 16

17

SRK & PR 18

Activity Models Although Equation of State models have proven to be very reliable in predicting the properties of most hydrocarbon based fluids over a wide range of operating conditions, their application has been limited to primarily non-polar or slightly polar components. Highly non-ideal systems are best modelled using Activity Models. The following Activity Model Property Packages are available: 19

20

Chao Seader Models The Chao Seader and Grayson Streed methods are older, semi empirical methods. The Grayson Streed correlation is an extension of the Chao Seader method with special emphasis on hydrogen. Only the equilibrium data produced by these correlations is used by HYSYS. The Lee-Kesler method is used for liquid and vapour enthalpies and entropies. 21

22

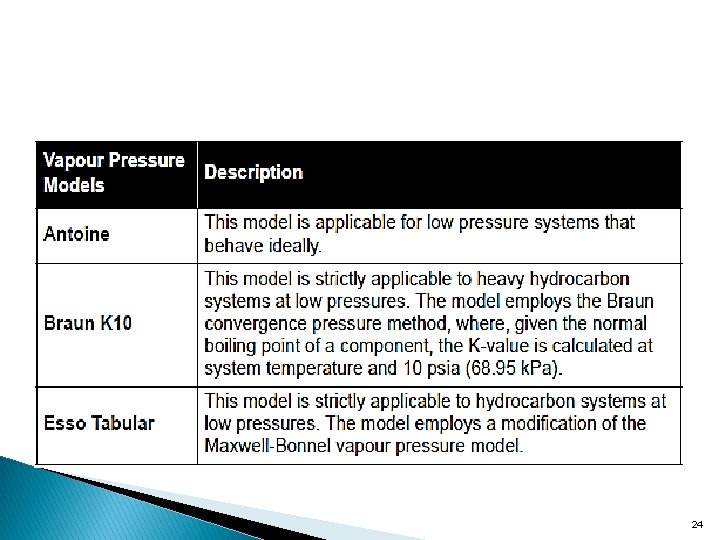
Vapour Pressure Models Vapour Pressure K-value models may be used for ideal mixtures at low pressures. Ideal mixtures include hydrocarbon systems and mixtures such as ketones and alcohols, where the liquid phase behaviour is approximately ideal. The models may also be used as first approximations for non-ideal systems: 23

24

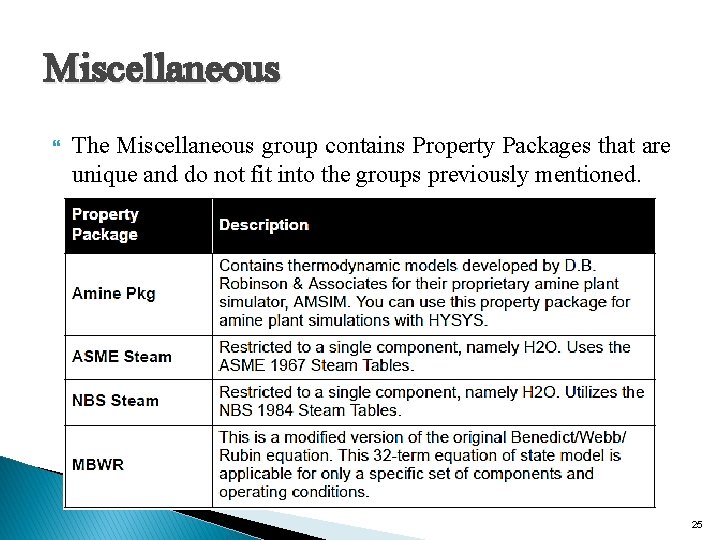
Miscellaneous The Miscellaneous group contains Property Packages that are unique and do not fit into the groups previously mentioned. 25

26