Specific Heat Q m c T Q Heat






- Slides: 26

Specific Heat Q = m c ΔT

Q • Heat absorbed by a material • Measured in Joules (J)

m • Mass of the material • Measured in grams (g)

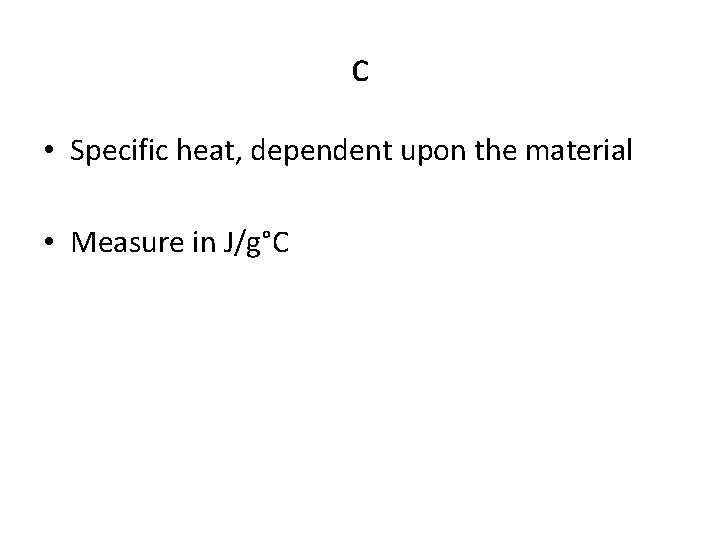
c • Specific heat, dependent upon the material • Measure in J/g°C

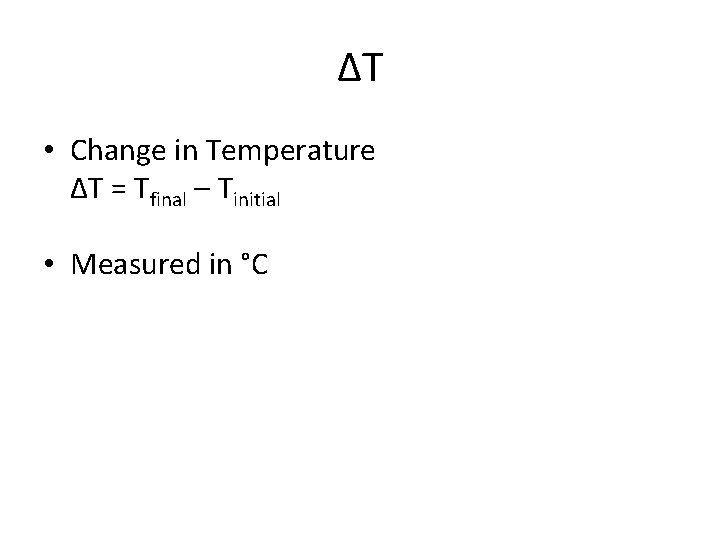
ΔT • Change in Temperature ΔT = Tfinal – Tinitial • Measured in °C

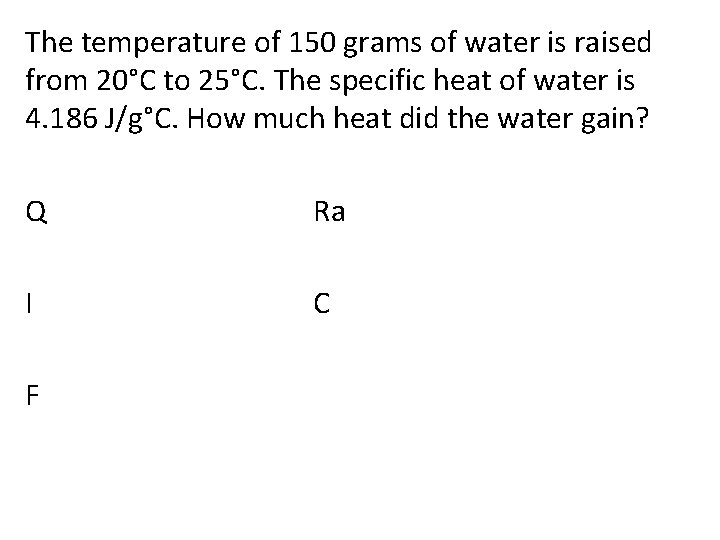
The temperature of 150 grams of water is raised from 20°C to 25°C. The specific heat of water is 4. 186 J/g°C. How much heat did the water gain? Q Ra I C F

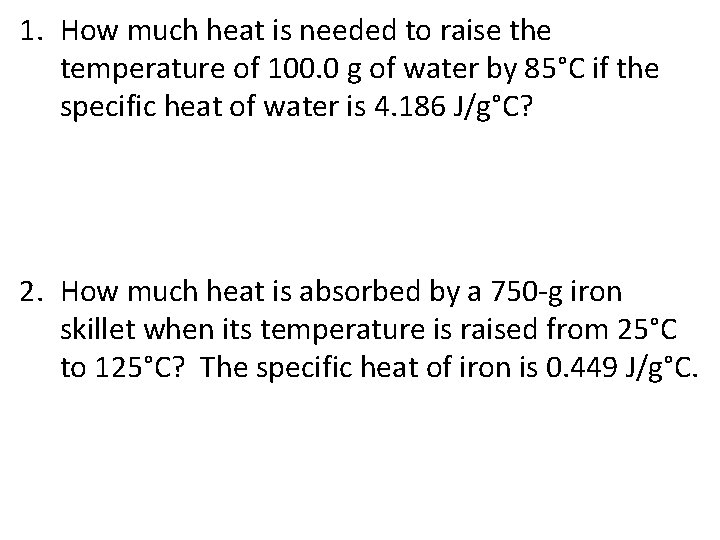
1. How much heat is needed to raise the temperature of 100. 0 g of water by 85°C if the specific heat of water is 4. 186 J/g°C? 2. How much heat is absorbed by a 750 -g iron skillet when its temperature is raised from 25°C to 125°C? The specific heat of iron is 0. 449 J/g°C.

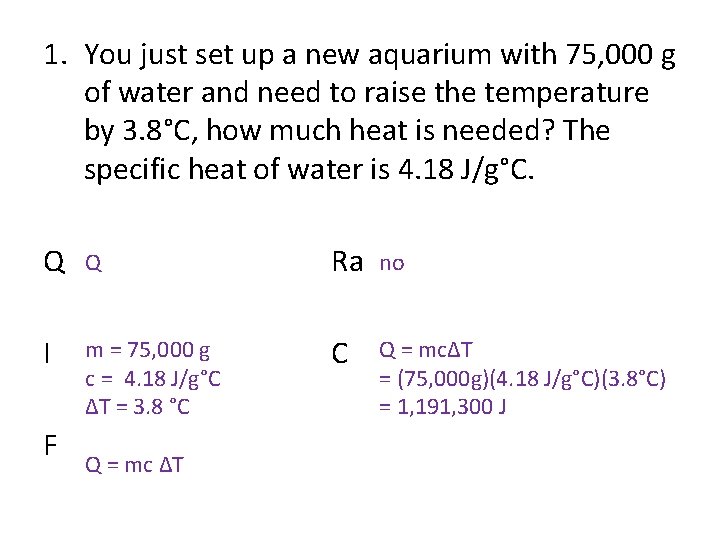
1. You just set up a new aquarium with 75, 000 g of water and need to raise the temperature by 3. 8°C, how much heat is needed? The specific heat of water is 4. 18 J/g°C. Q Q Ra no I m = 75, 000 g c = 4. 18 J/g°C ΔT = 3. 8 °C C Q = mcΔT = (75, 000 g)(4. 18 J/g°C)(3. 8°C) = 1, 191, 300 J F Q = mc ΔT

2. A jeweler is trying to release a diamond from its setting in a 10. 0 g silver ring. If the temperature has to change by 10. 0°C, how much heat is needed? The specific heat of silver is 0. 235 J/g°C. Q Q Ra no I m = 10. 0 g c = 0. 235 J/g°C ΔT = 10. 0 °C C Q = mcΔT F Q = mc ΔT = (10. 0 g)(0. 235 J/g°C)(10. 0°C) = 23. 5 J

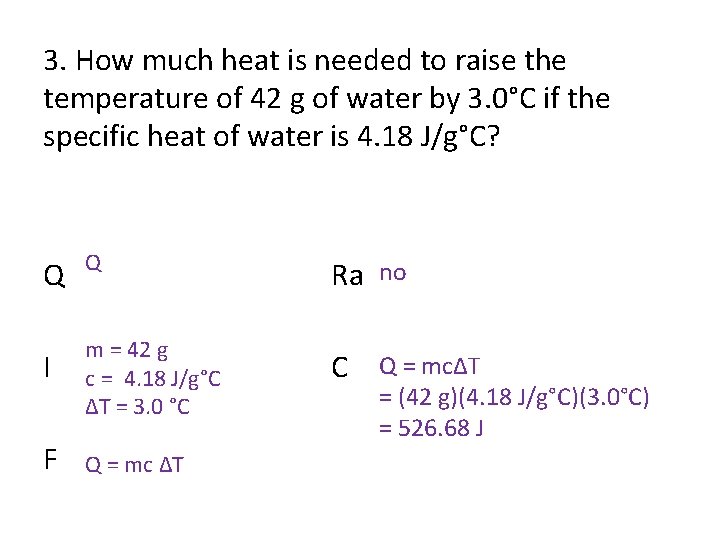
3. How much heat is needed to raise the temperature of 42 g of water by 3. 0°C if the specific heat of water is 4. 18 J/g°C? Q Q Ra no I m = 42 g c = 4. 18 J/g°C ΔT = 3. 0 °C C Q = mcΔT F Q = mc ΔT = (42 g)(4. 18 J/g°C)(3. 0°C) = 526. 68 J

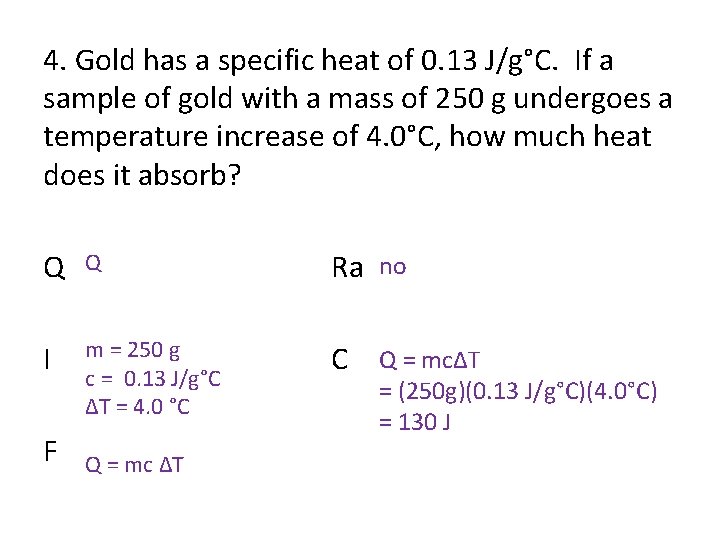
4. Gold has a specific heat of 0. 13 J/g°C. If a sample of gold with a mass of 250 g undergoes a temperature increase of 4. 0°C, how much heat does it absorb? Q Q Ra no I m = 250 g c = 0. 13 J/g°C ΔT = 4. 0 °C C Q = mcΔT F Q = mc ΔT = (250 g)(0. 13 J/g°C)(4. 0°C) = 130 J

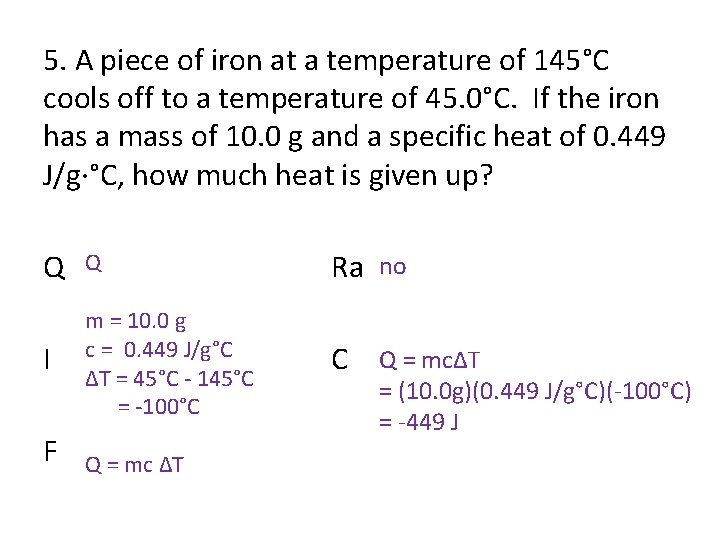
5. A piece of iron at a temperature of 145°C cools off to a temperature of 45. 0°C. If the iron has a mass of 10. 0 g and a specific heat of 0. 449 J/g·°C, how much heat is given up? Q Q Ra no I m = 10. 0 g c = 0. 449 J/g°C ΔT = 45°C - 145°C = -100°C C Q = mcΔT F Q = mc ΔT = (10. 0 g)(0. 449 J/g°C)(-100°C) = -449 J

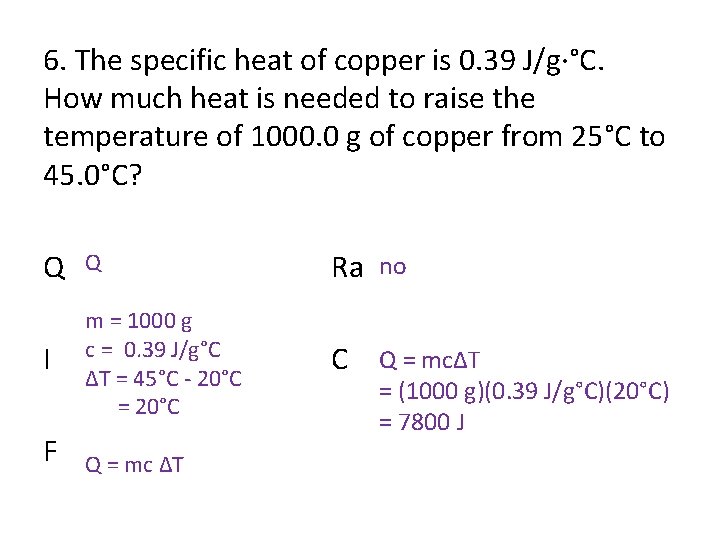
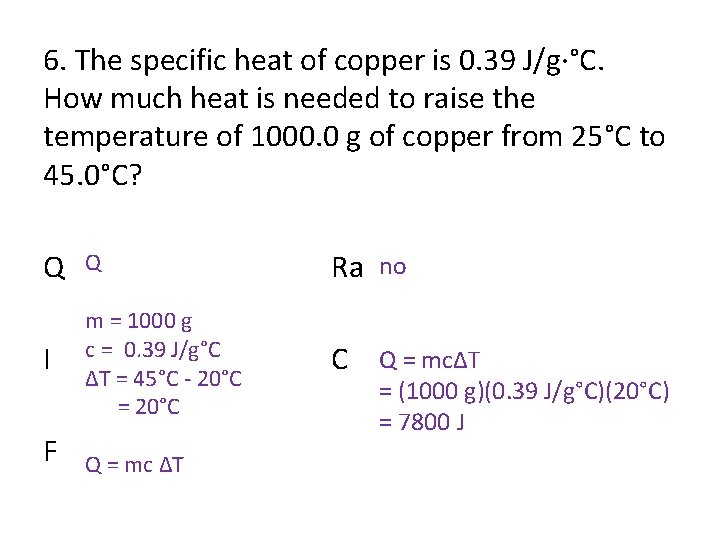
6. The specific heat of copper is 0. 39 J/g·°C. How much heat is needed to raise the temperature of 1000. 0 g of copper from 25°C to 45. 0°C? Q Q Ra no I m = 1000 g c = 0. 39 J/g°C ΔT = 45°C - 20°C = 20°C C Q = mcΔT F Q = mc ΔT = (1000 g)(0. 39 J/g°C)(20°C) = 7800 J

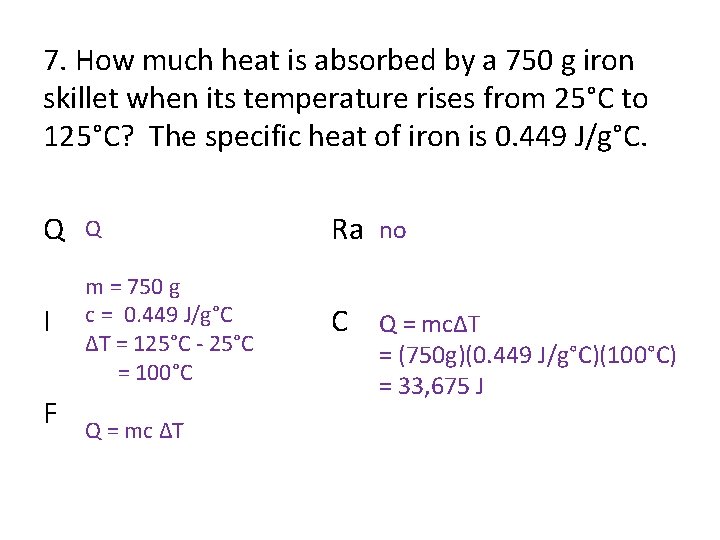
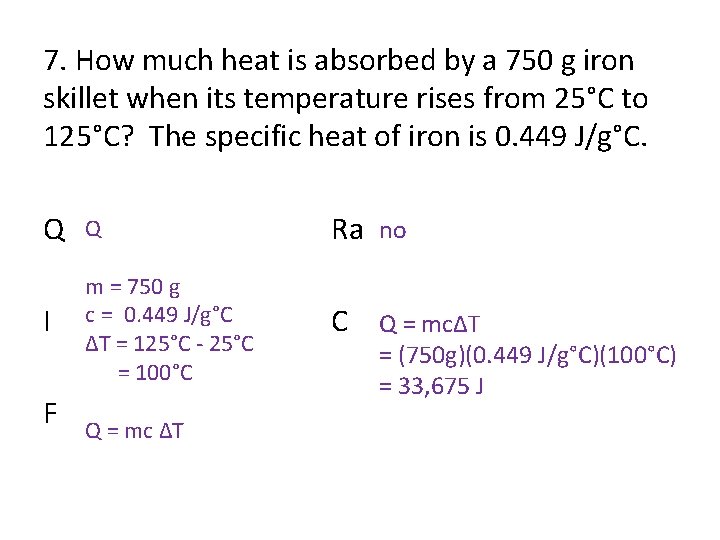
7. How much heat is absorbed by a 750 g iron skillet when its temperature rises from 25°C to 125°C? The specific heat of iron is 0. 449 J/g°C. Q Q Ra no I m = 750 g c = 0. 449 J/g°C ΔT = 125°C - 25°C = 100°C C Q = mcΔT F Q = mc ΔT = (750 g)(0. 449 J/g°C)(100°C) = 33, 675 J

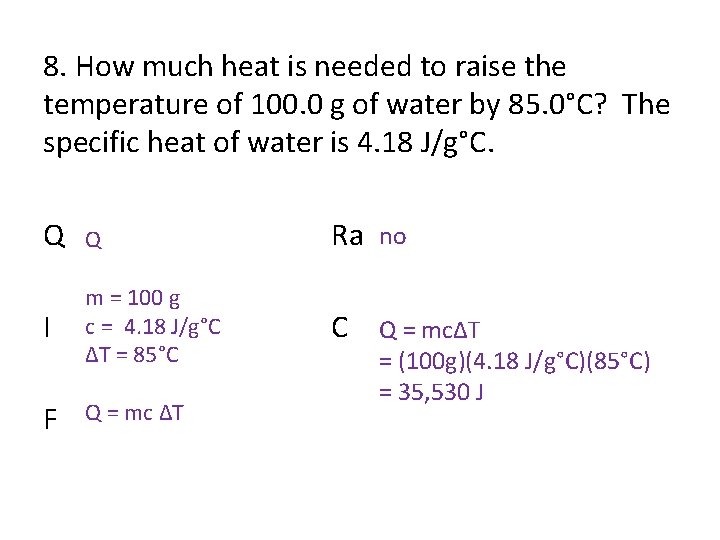
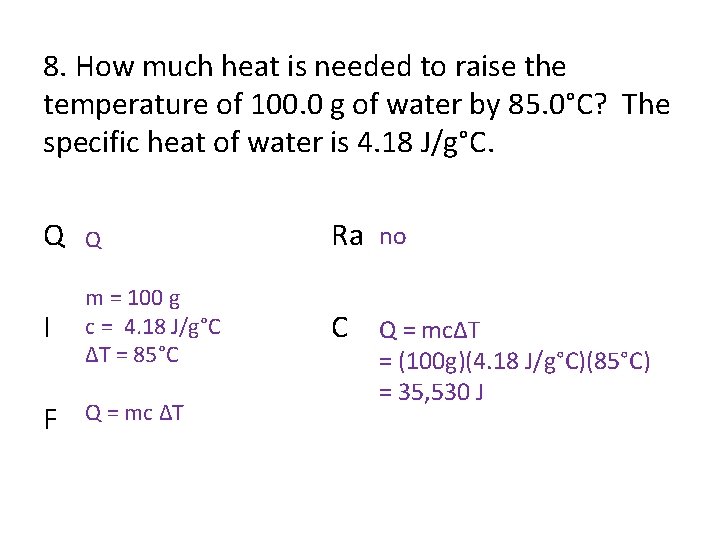
8. How much heat is needed to raise the temperature of 100. 0 g of water by 85. 0°C? The specific heat of water is 4. 18 J/g°C. Q Q Ra no I m = 100 g c = 4. 18 J/g°C ΔT = 85°C C Q = mcΔT F Q = mc ΔT = (100 g)(4. 18 J/g°C)(85°C) = 35, 530 J

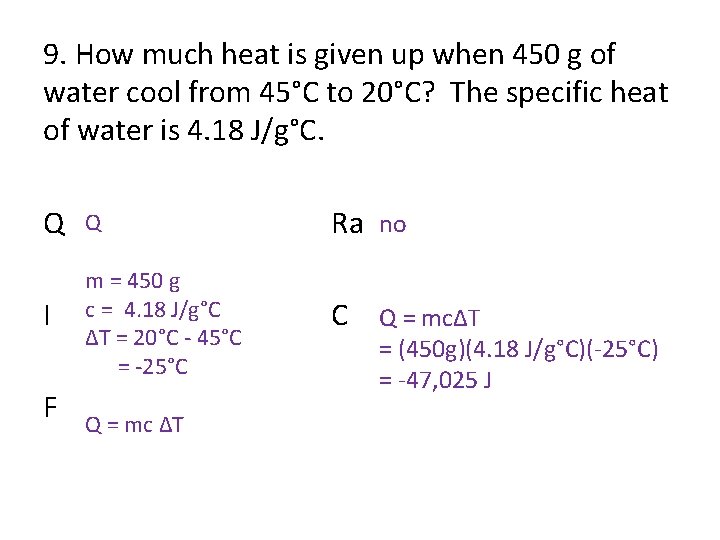
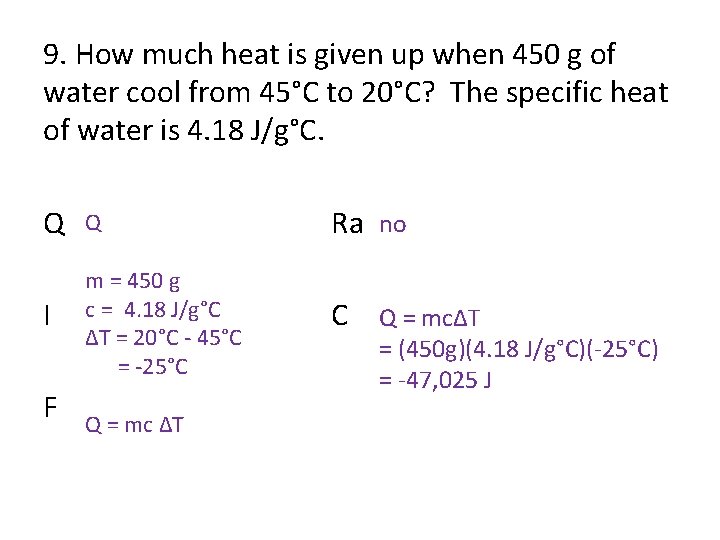
9. How much heat is given up when 450 g of water cool from 45°C to 20°C? The specific heat of water is 4. 18 J/g°C. Q Q Ra no I m = 450 g c = 4. 18 J/g°C ΔT = 20°C - 45°C = -25°C C Q = mcΔT F Q = mc ΔT = (450 g)(4. 18 J/g°C)(-25°C) = -47, 025 J

Activator Q = mcΔT What does each variable stand for?

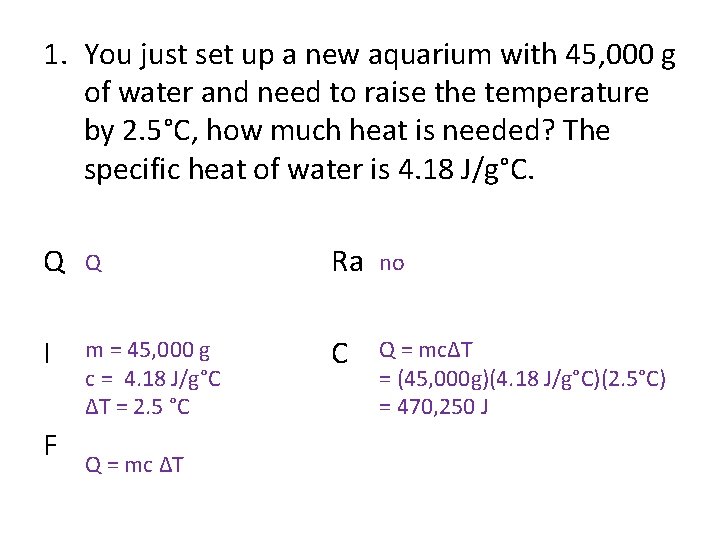
1. You just set up a new aquarium with 45, 000 g of water and need to raise the temperature by 2. 5°C, how much heat is needed? The specific heat of water is 4. 18 J/g°C. Q Q Ra no I m = 45, 000 g c = 4. 18 J/g°C ΔT = 2. 5 °C C Q = mcΔT = (45, 000 g)(4. 18 J/g°C)(2. 5°C) = 470, 250 J F Q = mc ΔT

2. A jeweler is trying to release a diamond from its setting in a 8. 0 g silver ring. If the temperature has to change by 12. 5°C, how much heat is needed? The specific heat of silver is 0. 235 J/g°C. Q Q Ra no I m = 8. 0 g c = 0. 235 J/g°C ΔT = 12. 5 °C C Q = mcΔT F Q = mc ΔT = (8. 0 g)(0. 235 J/g°C)(12. 5°C) = 23. 5 J

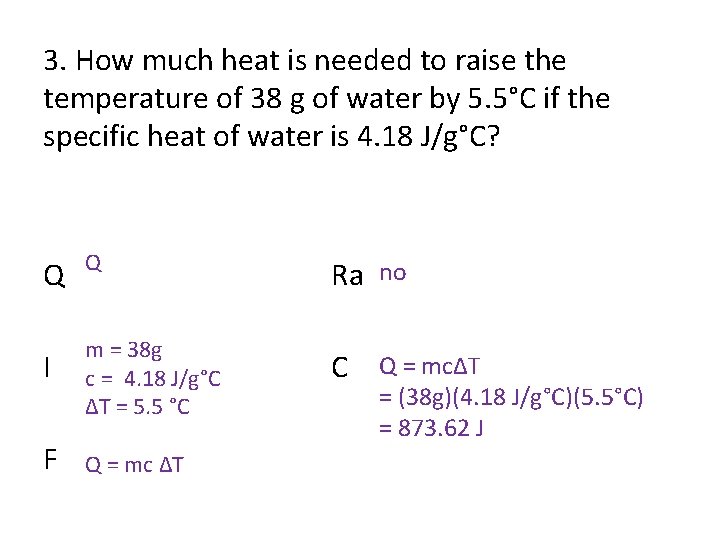
3. How much heat is needed to raise the temperature of 38 g of water by 5. 5°C if the specific heat of water is 4. 18 J/g°C? Q Q Ra no I m = 38 g c = 4. 18 J/g°C ΔT = 5. 5 °C C Q = mcΔT F Q = mc ΔT = (38 g)(4. 18 J/g°C)(5. 5°C) = 873. 62 J

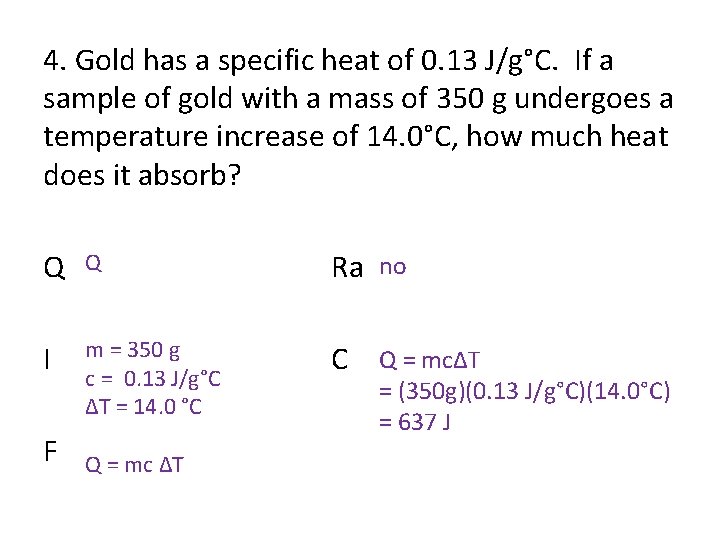
4. Gold has a specific heat of 0. 13 J/g°C. If a sample of gold with a mass of 350 g undergoes a temperature increase of 14. 0°C, how much heat does it absorb? Q Q Ra no I m = 350 g c = 0. 13 J/g°C ΔT = 14. 0 °C C Q = mcΔT F Q = mc ΔT = (350 g)(0. 13 J/g°C)(14. 0°C) = 637 J

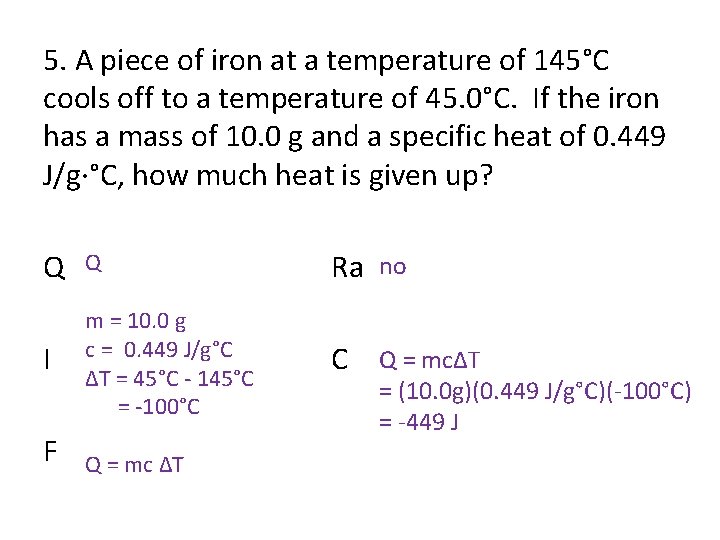
5. A piece of iron at a temperature of 145°C cools off to a temperature of 45. 0°C. If the iron has a mass of 10. 0 g and a specific heat of 0. 449 J/g·°C, how much heat is given up? Q Q Ra no I m = 10. 0 g c = 0. 449 J/g°C ΔT = 45°C - 145°C = -100°C C Q = mcΔT F Q = mc ΔT = (10. 0 g)(0. 449 J/g°C)(-100°C) = -449 J

6. The specific heat of copper is 0. 39 J/g·°C. How much heat is needed to raise the temperature of 1000. 0 g of copper from 25°C to 45. 0°C? Q Q Ra no I m = 1000 g c = 0. 39 J/g°C ΔT = 45°C - 20°C = 20°C C Q = mcΔT F Q = mc ΔT = (1000 g)(0. 39 J/g°C)(20°C) = 7800 J

7. How much heat is absorbed by a 750 g iron skillet when its temperature rises from 25°C to 125°C? The specific heat of iron is 0. 449 J/g°C. Q Q Ra no I m = 750 g c = 0. 449 J/g°C ΔT = 125°C - 25°C = 100°C C Q = mcΔT F Q = mc ΔT = (750 g)(0. 449 J/g°C)(100°C) = 33, 675 J

8. How much heat is needed to raise the temperature of 100. 0 g of water by 85. 0°C? The specific heat of water is 4. 18 J/g°C. Q Q Ra no I m = 100 g c = 4. 18 J/g°C ΔT = 85°C C Q = mcΔT F Q = mc ΔT = (100 g)(4. 18 J/g°C)(85°C) = 35, 530 J

9. How much heat is given up when 450 g of water cool from 45°C to 20°C? The specific heat of water is 4. 18 J/g°C. Q Q Ra no I m = 450 g c = 4. 18 J/g°C ΔT = 20°C - 45°C = -25°C C Q = mcΔT F Q = mc ΔT = (450 g)(4. 18 J/g°C)(-25°C) = -47, 025 J