Specific Heat IB 1 Chemistry 10 Specific Heat

Specific Heat IB 1 Chemistry

10 Specific Heat • Adding Energy to a material Causes the • Temperature to go up. • Taking energy away from a substance causes the temp. to • Go down!
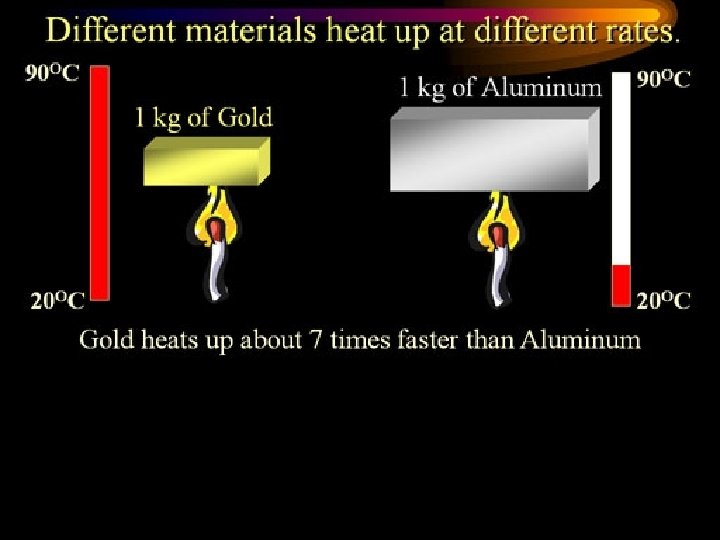
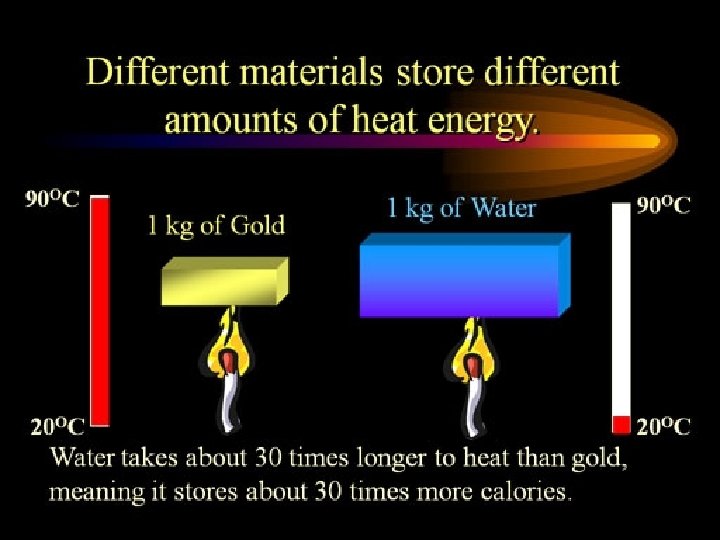

15 Specific Heat • The amount of energy required to raise the temperature of a material (substance). • It takes different amts of energy to make the same temp change in different substances. • We call the amt required: Specific Heat!

16 Specific Heat of water • The Cp is high because H 2 O mols. form strong bonds w/each other. • It takes a lot of energy to break the bonds so that the molecules can then start to move around faster (HEAT UP).

17 Example: Specific Heat of Water • Cp = 4, 184 Joules of energy to raise the temperature of 1 kg 1°C. • video clip Why Cp? Cp Stands for “Heat Capacity”
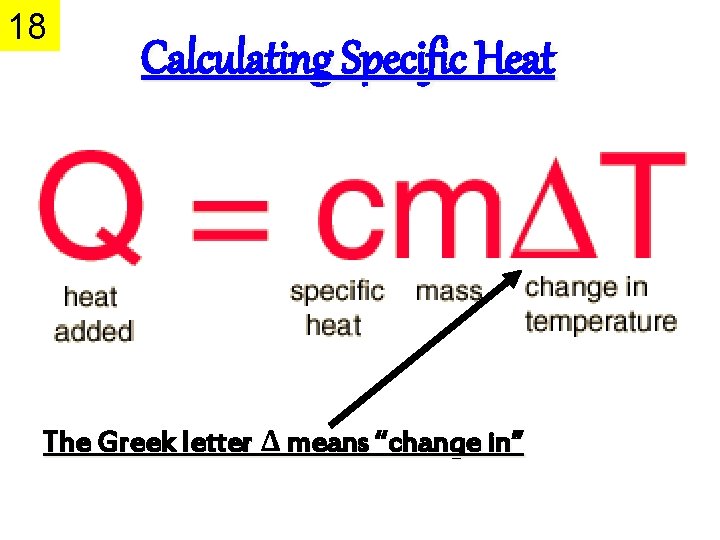
18 Calculating Specific Heat The Greek letter Δ means “change in”
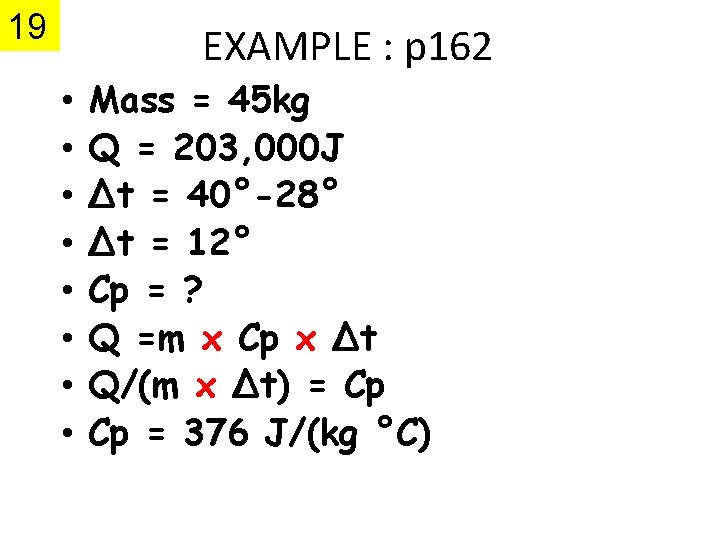
19 EXAMPLE : p 162 • • Mass = 45 kg Q = 203, 000 J Δt = 40°-28° Δt = 12° Cp = ? Q =m x Cp x Δt Q/(m x Δt) = Cp Cp = 376 J/(kg °C)
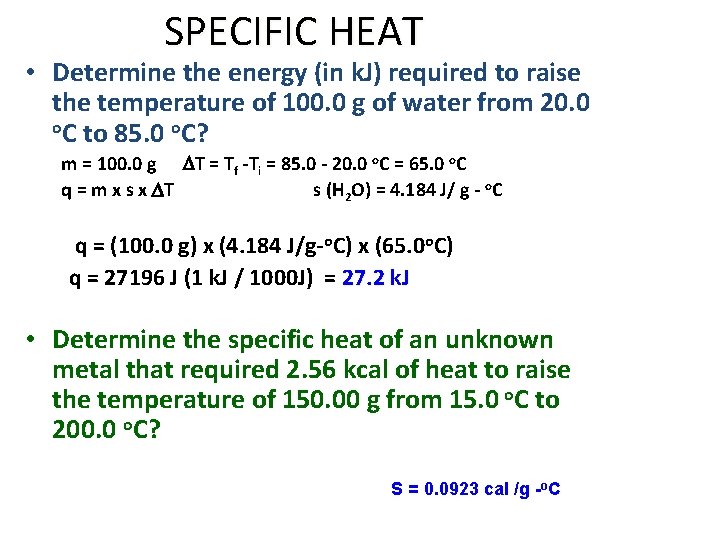
SPECIFIC HEAT • Determine the energy (in k. J) required to raise the temperature of 100. 0 g of water from 20. 0 o. C to 85. 0 o. C? m = 100. 0 g DT = Tf -Ti = 85. 0 - 20. 0 o. C = 65. 0 o. C q = m x s x DT s (H 2 O) = 4. 184 J/ g - o. C q = (100. 0 g) x (4. 184 J/g-o. C) x (65. 0 o. C) q = 27196 J (1 k. J / 1000 J) = 27. 2 k. J • Determine the specific heat of an unknown metal that required 2. 56 kcal of heat to raise the temperature of 150. 00 g from 15. 0 o. C to 200. 0 o. C? S = 0. 0923 cal /g -o. C
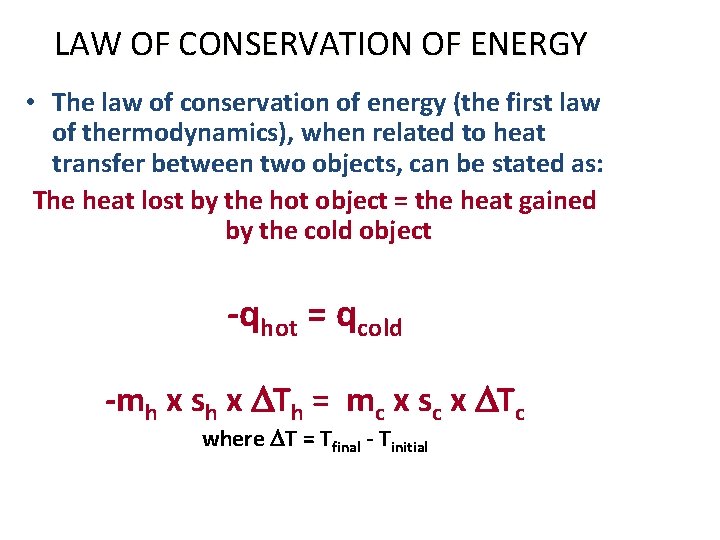
LAW OF CONSERVATION OF ENERGY • The law of conservation of energy (the first law of thermodynamics), when related to heat transfer between two objects, can be stated as: The heat lost by the hot object = the heat gained by the cold object -qhot = qcold -mh x sh x DTh = mc x sc x DTc where DT = Tfinal - Tinitial
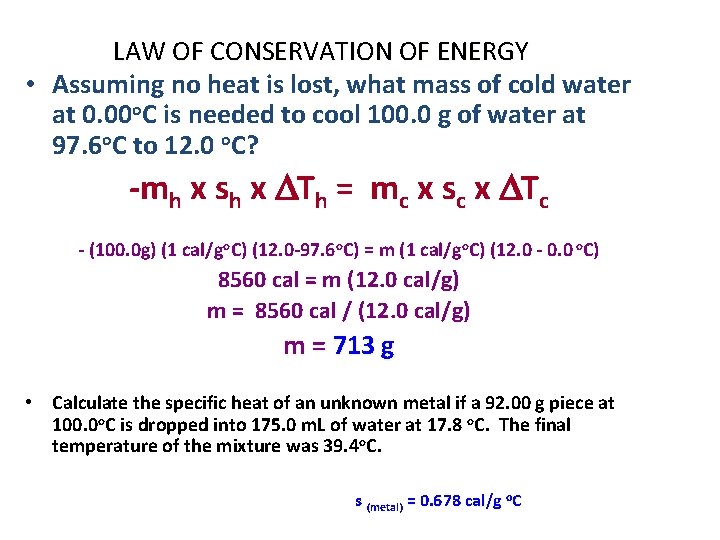
LAW OF CONSERVATION OF ENERGY • Assuming no heat is lost, what mass of cold water at 0. 00 o. C is needed to cool 100. 0 g of water at 97. 6 o. C to 12. 0 o. C? -mh x sh x DTh = mc x sc x DTc - (100. 0 g) (1 cal/go. C) (12. 0 -97. 6 o. C) = m (1 cal/go. C) (12. 0 - 0. 0 o. C) 8560 cal = m (12. 0 cal/g) m = 8560 cal / (12. 0 cal/g) m = 713 g • Calculate the specific heat of an unknown metal if a 92. 00 g piece at 100. 0 o. C is dropped into 175. 0 m. L of water at 17. 8 o. C. The final temperature of the mixture was 39. 4 o. C. s (metal) = 0. 678 cal/g o. C
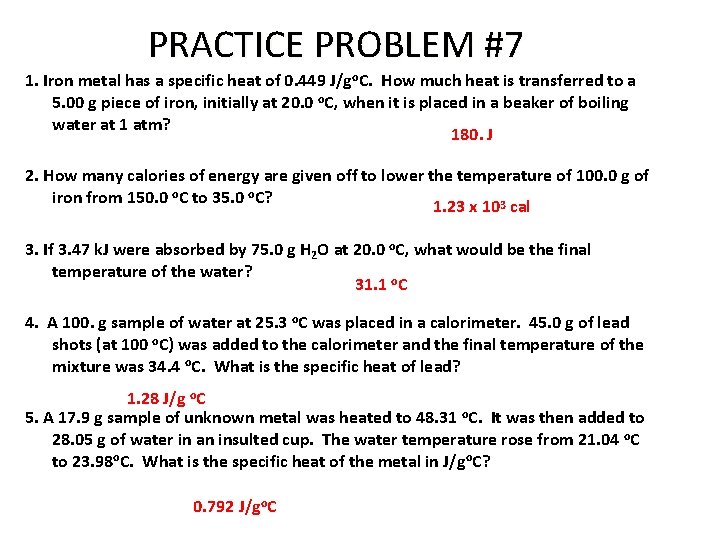
PRACTICE PROBLEM #7 1. Iron metal has a specific heat of 0. 449 J/go. C. How much heat is transferred to a 5. 00 g piece of iron, initially at 20. 0 o. C, when it is placed in a beaker of boiling water at 1 atm? 180. J 2. How many calories of energy are given off to lower the temperature of 100. 0 g of iron from 150. 0 o. C to 35. 0 o. C? 1. 23 x 103 cal 3. If 3. 47 k. J were absorbed by 75. 0 g H 2 O at 20. 0 o. C, what would be the final temperature of the water? 31. 1 o. C 4. A 100. g sample of water at 25. 3 o. C was placed in a calorimeter. 45. 0 g of lead shots (at 100 o. C) was added to the calorimeter and the final temperature of the mixture was 34. 4 o. C. What is the specific heat of lead? 1. 28 J/g o. C 5. A 17. 9 g sample of unknown metal was heated to 48. 31 o. C. It was then added to 28. 05 g of water in an insulted cup. The water temperature rose from 21. 04 o. C to 23. 98 o. C. What is the specific heat of the metal in J/go. C? 0. 792 J/go. C
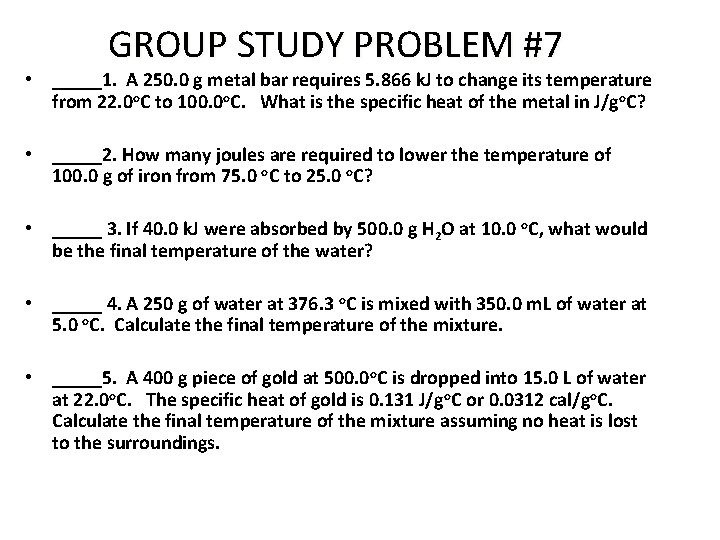
GROUP STUDY PROBLEM #7 • _____1. A 250. 0 g metal bar requires 5. 866 k. J to change its temperature from 22. 0 o. C to 100. 0 o. C. What is the specific heat of the metal in J/go. C? • _____2. How many joules are required to lower the temperature of 100. 0 g of iron from 75. 0 o. C to 25. 0 o. C? • _____ 3. If 40. 0 k. J were absorbed by 500. 0 g H 2 O at 10. 0 o. C, what would be the final temperature of the water? • _____ 4. A 250 g of water at 376. 3 o. C is mixed with 350. 0 m. L of water at 5. 0 o. C. Calculate the final temperature of the mixture. • _____5. A 400 g piece of gold at 500. 0 o. C is dropped into 15. 0 L of water at 22. 0 o. C. The specific heat of gold is 0. 131 J/go. C or 0. 0312 cal/go. C. Calculate the final temperature of the mixture assuming no heat is lost to the surroundings.
- Slides: 14