Specific Heat Capacity Objectives What is the difference

Specific Heat Capacity Objectives • What is the difference between heat and temperature? • What is specific heat capacity? • How is it calculated? • How is it useful? Why do you have to be careful with one of these?

Caution: Hot Apple Pie! • Have you bitten into an apple pie and burnt your mouth? • The outside is warm but the inside is very hot. • It was all cooked together so why does this happen? It’s because the filling has a higher heat capacity than the pastry.
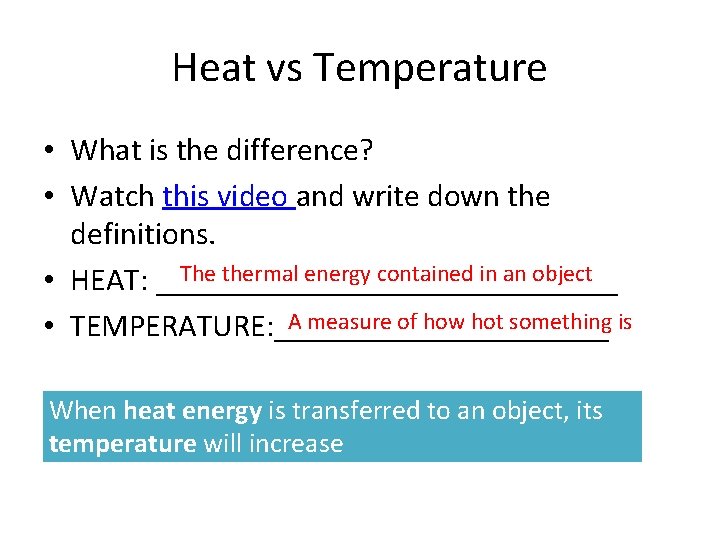
Heat vs Temperature • What is the difference? • Watch this video and write down the definitions. The thermal energy contained in an object • HEAT: _______________ A measure of how hot something is • TEMPERATURE: ___________ When heat energy is transferred to an object, its temperature will increase
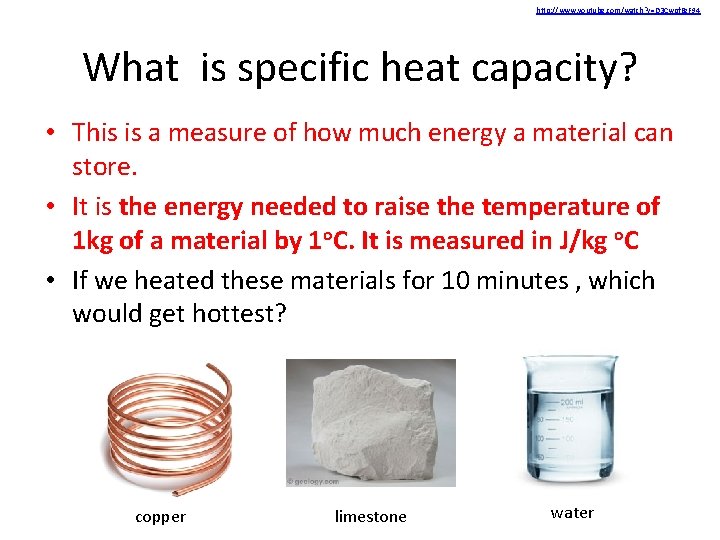
http: //www. youtube. com/watch? v=D 3 Cwpf. Bz. F 94 What is specific heat capacity? • This is a measure of how much energy a material can store. • It is the energy needed to raise the temperature of 1 kg of a material by 1 o. C. It is measured in J/kg o. C • If we heated these materials for 10 minutes , which would get hottest? copper limestone water

SHC of different materials 82 o. C 65 o. C 43 o. C • The copper became hottest because it has the lowest SHC. • The water has the highest SHC as it absorbed the energy without becoming very hot.
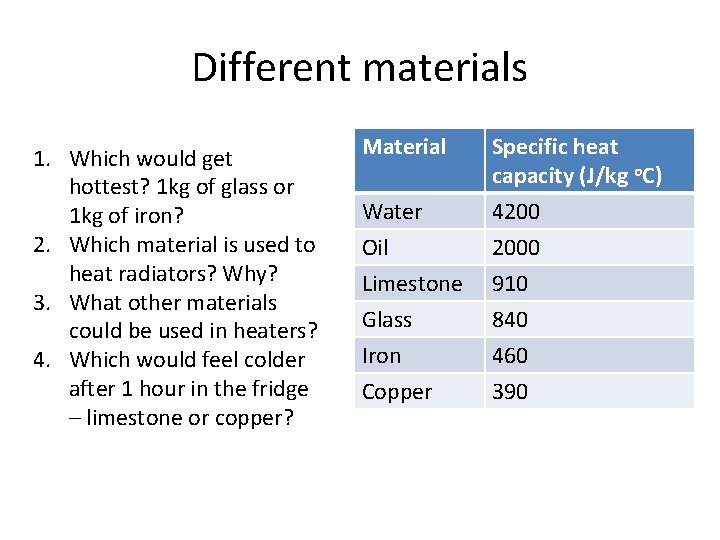
Different materials 1. Which would get hottest? 1 kg of glass or 1 kg of iron? 2. Which material is used to heat radiators? Why? 3. What other materials could be used in heaters? 4. Which would feel colder after 1 hour in the fridge – limestone or copper? Material Specific heat capacity (J/kg o. C) Water Oil 4200 2000 Limestone Glass Iron Copper 910 840 460 390
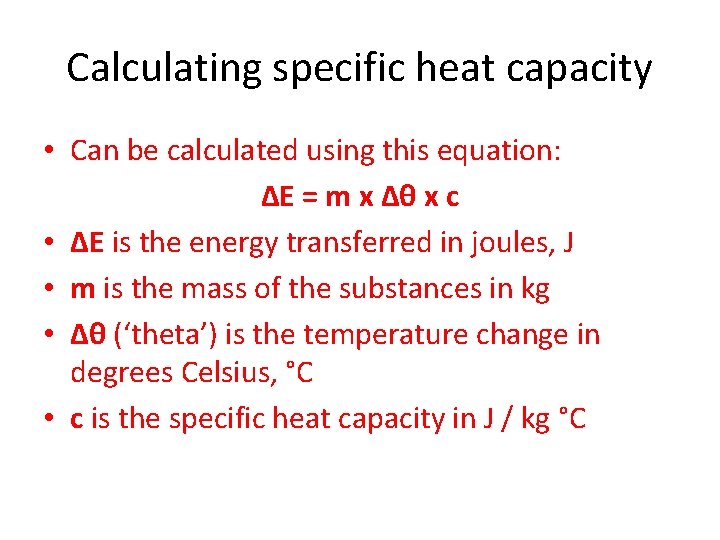
Calculating specific heat capacity • Can be calculated using this equation: ΔE = m x Δθ x c • ΔE is the energy transferred in joules, J • m is the mass of the substances in kg • Δθ (‘theta’) is the temperature change in degrees Celsius, °C • c is the specific heat capacity in J / kg °C
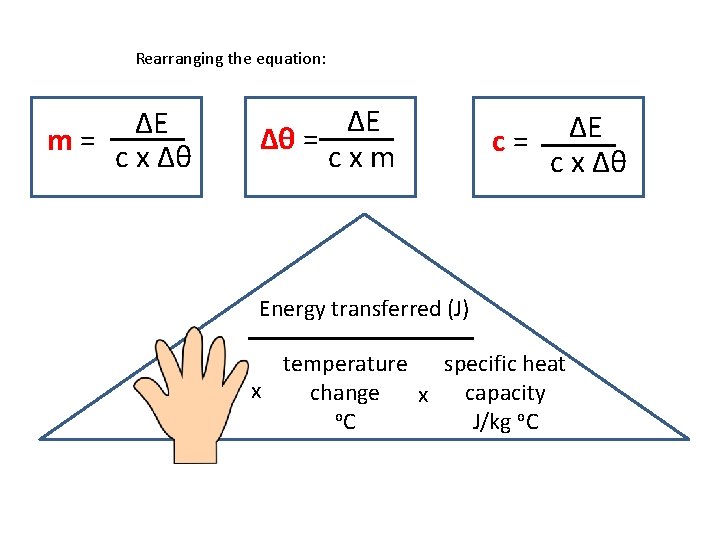
Rearranging the equation: ΔE m= c x Δθ ΔE Δθ = cxm c= ΔE c x Δθ Energy transferred (J) mass kg specific heat temperature x capacity change x o. C J/kg o. C
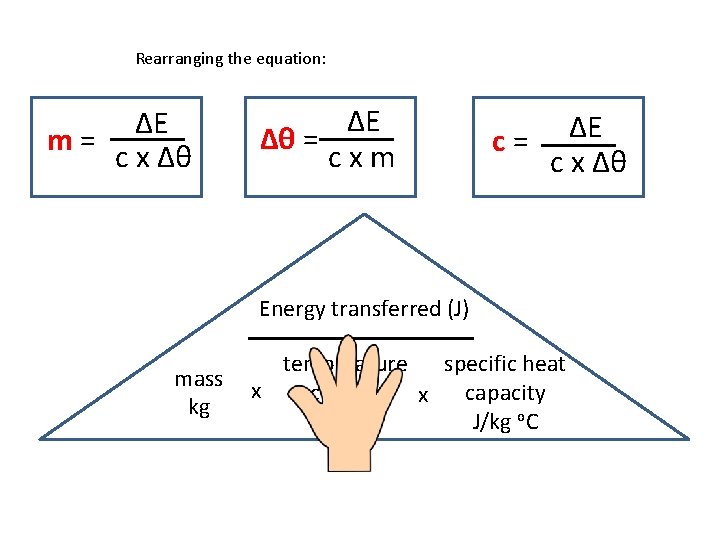
Rearranging the equation: ΔE m= c x Δθ ΔE Δθ = cxm c= ΔE c x Δθ Energy transferred (J) mass kg specific heat temperature x capacity change x o. C J/kg o. C
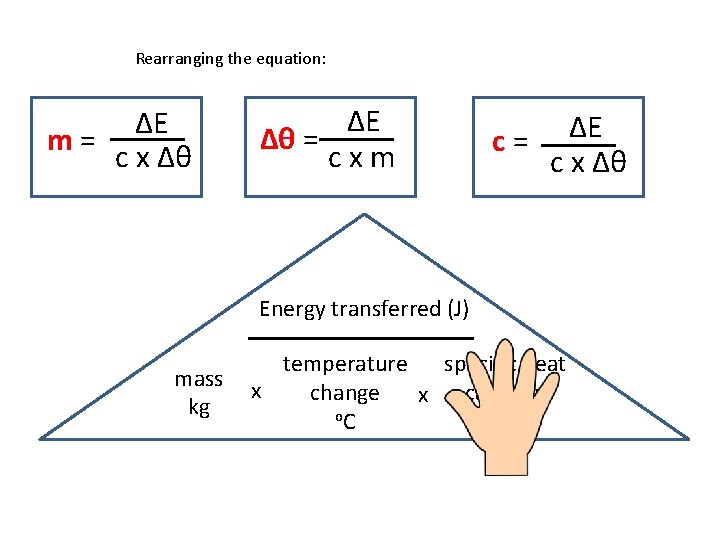
Rearranging the equation: ΔE m= c x Δθ ΔE Δθ = cxm c= ΔE c x Δθ Energy transferred (J) mass kg specific heat temperature x capacity change x o. C J/kg o. C
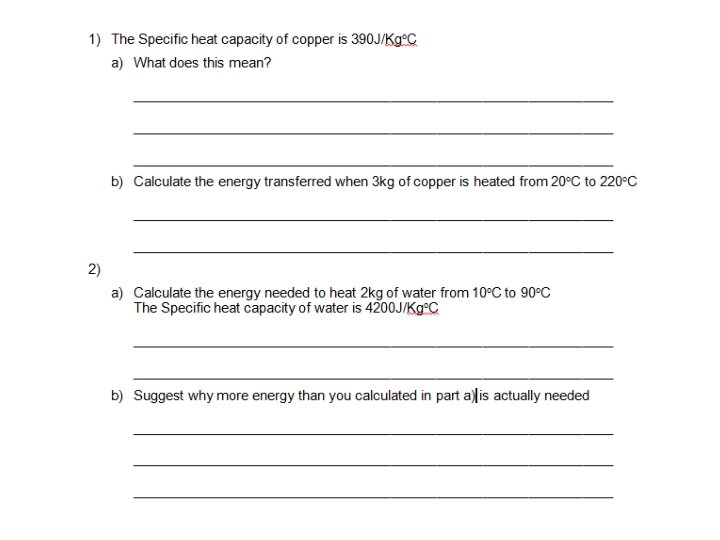
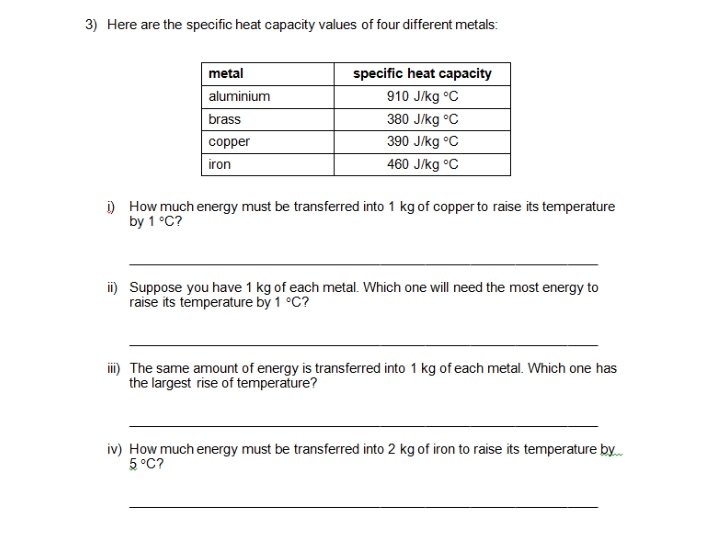
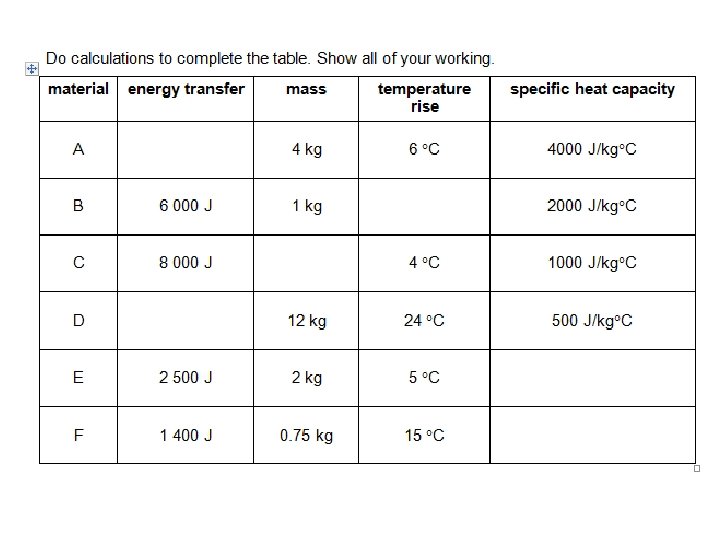

Examples – show your working E=mxθxc 1. For example, how much energy must be transferred to raise the temperature of 2 kg of water from 20°C to 30°C? 2. How much energy is needed to raise the temperature of 1 kg of glass from 30°C to 45°C? 3. How much energy is needed to increase the temperature of 500 g of lead from 20°C to 45°C? The specific heat capacity of lead is 128 J/kg °C.

Frying an egg in paper • Can you fry an egg in newspaper? • Watch the clip. Can you explain how it works?

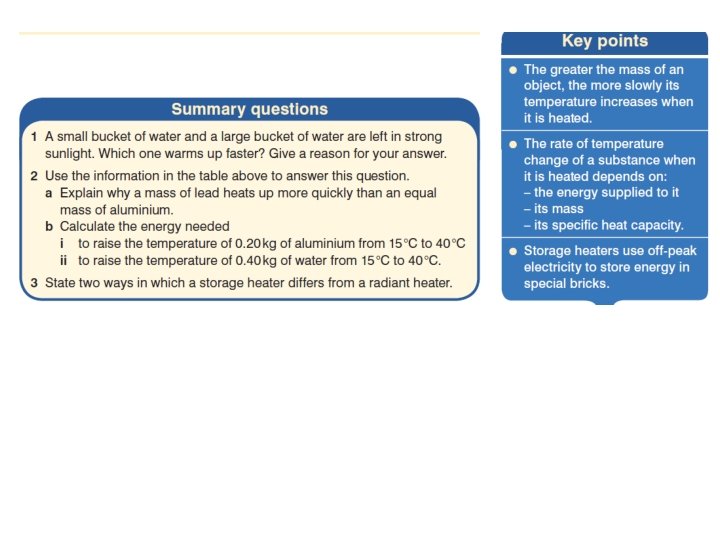
- Slides: 17