Specific Heat Calorimetry Calculating Heat Transferred Q m






- Slides: 12

Specific Heat Calorimetry

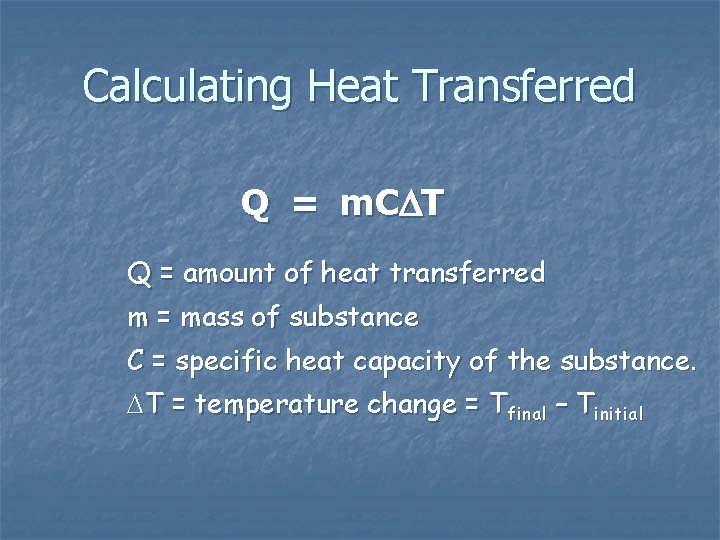
Calculating Heat Transferred Q = m. C T Q = amount of heat transferred m = mass of substance C = specific heat capacity of the substance. T = temperature change = Tfinal – Tinitial

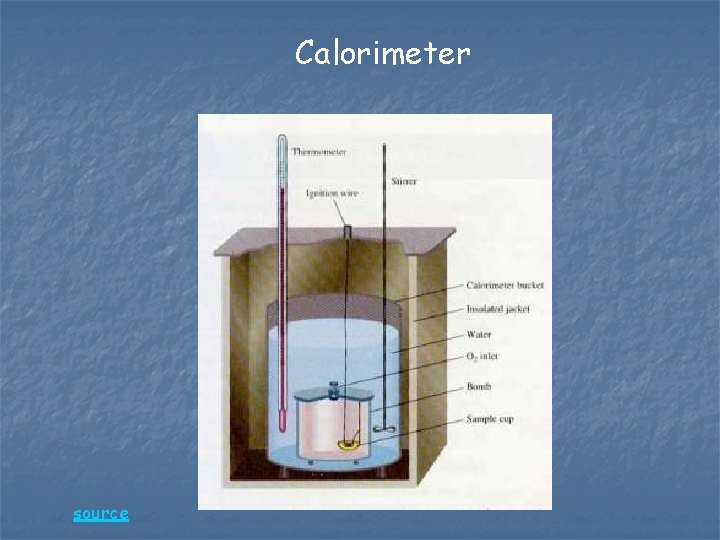
Calorimeter source

Another calorimeter source

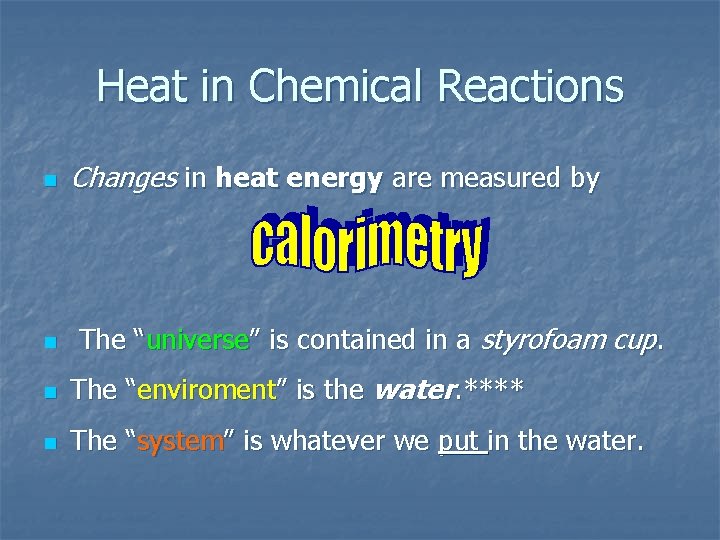
Heat in Chemical Reactions n n Changes in heat energy are measured by The “universe” is contained in a styrofoam cup. n The “enviroment” is the water. **** n The “system” is whatever we put in the water.

Calorimetry Energy lost = Energy gained Difficult to monitor the “system. ” Easy to monitor the “environment” – that’s the water! Energy lost/gained by environment = Energy gained/lost by system

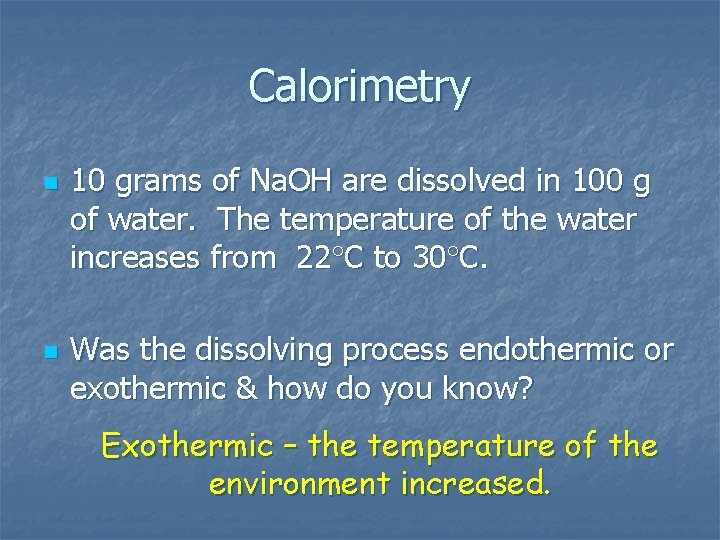
Calorimetry n n 10 grams of Na. OH are dissolved in 100 g of water. The temperature of the water increases from 22 C to 30 C. Was the dissolving process endothermic or exothermic & how do you know? Exothermic – the temperature of the environment increased.

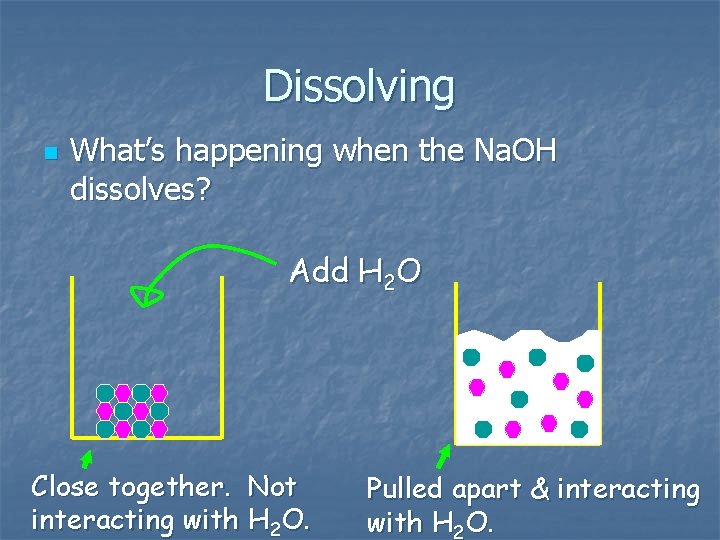
Dissolving n What’s happening when the Na. OH dissolves? Add H 2 O Close together. Not interacting with H 2 O. Pulled apart & interacting with H 2 O.

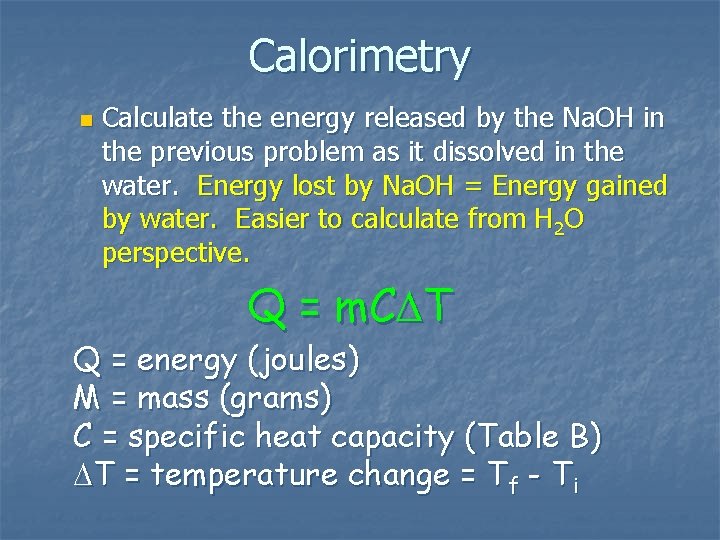
Calorimetry n Calculate the energy released by the Na. OH in the previous problem as it dissolved in the water. Energy lost by Na. OH = Energy gained by water. Easier to calculate from H 2 O perspective. Q = m. C T Q = energy (joules) M = mass (grams) C = specific heat capacity (Table B) T = temperature change = Tf - Ti

Calorimetry & Q = m. C T n n n The temperature of the water increased from 22 C to 30 C -22 C = 8 C = T What mass? Well, the temperature change was for the water, so you want the mass of the water. m = 100 g. Same goes for specific heat capacity. We’re going to calculate the heat absorbed by the water. CH 20 = 4. 18 J/g

Q = m. C T n. Q = 100 g X 4. 18 J/g X 8 C n. Q = 3344 Joules.

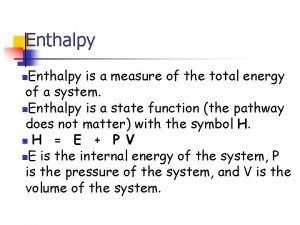
Expressing Heat Changes n n The heat content of a system at constant pressure is the same as a property called Heat released or absorbed (Q) by a reaction at constant pressure is the same as a change in enthalpy (r. H), therefore, Q = r. H.
 Optical rotation formula
Optical rotation formula Heat energy is transferred by conduction whenever molecules
Heat energy is transferred by conduction whenever molecules Radiation convection conduction
Radiation convection conduction Heat moves from
Heat moves from How is heat transferred in the mantle
How is heat transferred in the mantle 3 ways heat is transferred
3 ways heat is transferred Specific heat capacity table pdf
Specific heat capacity table pdf Latent heat and specific latent heat
Latent heat and specific latent heat Specific gravity units g/ml
Specific gravity units g/ml Specific gravity units g/ml
Specific gravity units g/ml Microscale combustion calorimetry
Microscale combustion calorimetry Molar heat capacity symbol
Molar heat capacity symbol Enthalpy and calorimetry
Enthalpy and calorimetry