Some reactions are Oxidation Reduction Reactions OxidationReduction also







- Slides: 19

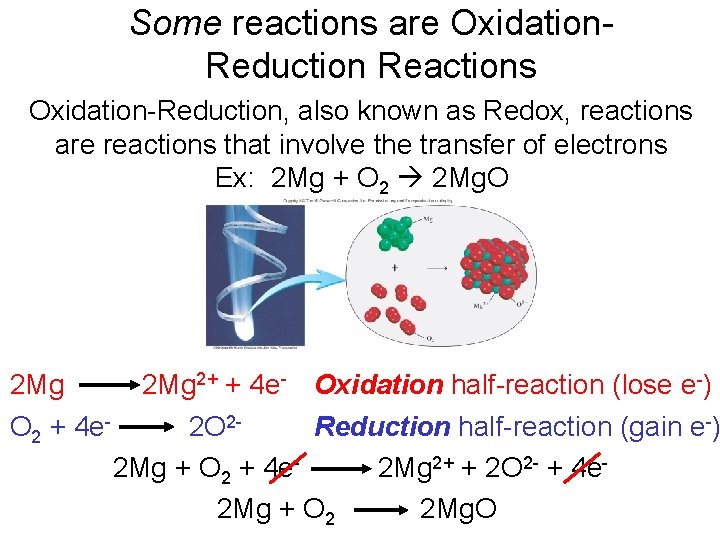
Some reactions are Oxidation. Reduction Reactions Oxidation-Reduction, also known as Redox, reactions are reactions that involve the transfer of electrons Ex: 2 Mg + O 2 2 Mg. O 2 Mg 2+ + 4 e- Oxidation half-reaction (lose e-) O 2 + 4 e 2 O 2 Reduction half-reaction (gain e-) 2 Mg + O 2 + 4 e 2 Mg 2+ + 2 O 2 - + 4 e 2 Mg + O 2 2 Mg. O

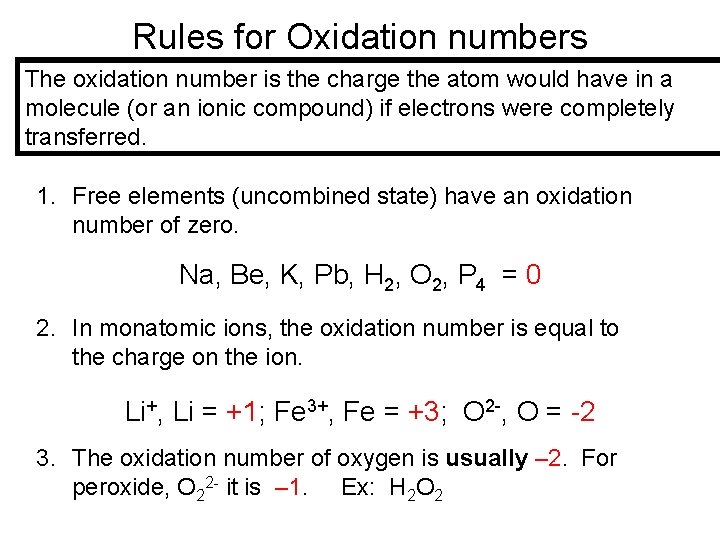
Rules for Oxidation numbers The oxidation number is the charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na, Be, K, Pb, H 2, O 2, P 4 = 0 2. In monatomic ions, the oxidation number is equal to the charge on the ion. Li+, Li = +1; Fe 3+, Fe = +3; O 2 -, O = -2 3. The oxidation number of oxygen is usually – 2. For peroxide, O 22 - it is – 1. Ex: H 2 O 2

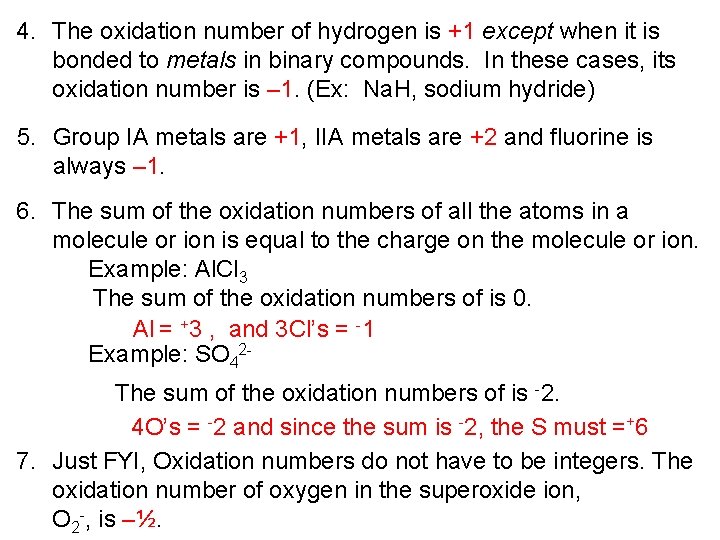
4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds. In these cases, its oxidation number is – 1. (Ex: Na. H, sodium hydride) 5. Group IA metals are +1, IIA metals are +2 and fluorine is always – 1. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. Example: Al. Cl 3 The sum of the oxidation numbers of is 0. Al = +3 , and 3 Cl’s = -1 Example: SO 42 The sum of the oxidation numbers of is -2. 4 O’s = -2 and since the sum is -2, the S must =+6 7. Just FYI, Oxidation numbers do not have to be integers. The oxidation number of oxygen in the superoxide ion, O 2 -, is –½.

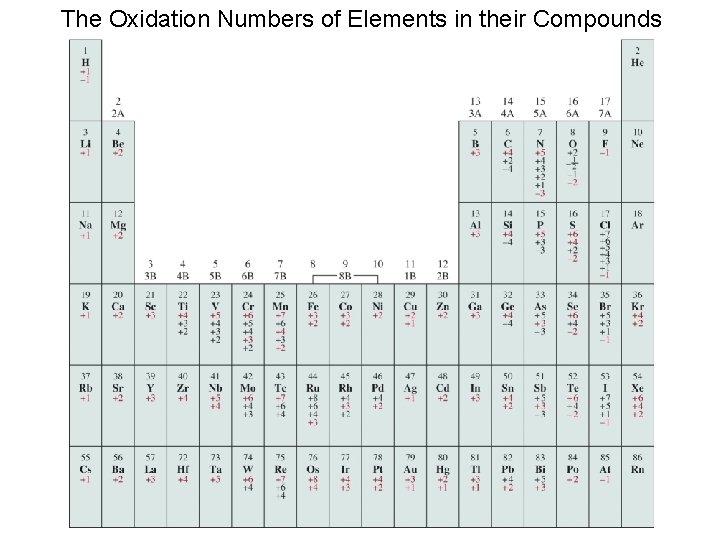
The Oxidation Numbers of Elements in their Compounds

Example Assign oxidation numbers to all the elements in the following compounds or ion: (a) Li 2 O (a) HNO 3 (b)

Predicting special reactions

1. metal + nonmetal binary salt n A piece of lithium metal is dropped into a container of nitrogen gas. 6 Li + N 2 2 Li 3 N

Reverse Reaction binary salt metal + nonmetal n Aluminum chloride decomposes in a vacuum. 2 Al. Cl 3→ 2 Al + 3 Cl 2

2. Metal oxide + water metal hydroxide (a base!) n Solid sodium oxide is added to water Na 2 O + HOH 2 Na. OH n Solid magnesium oxide is added to water Mg. O(s) + H 2 O(l) → Mg(OH)2(s)

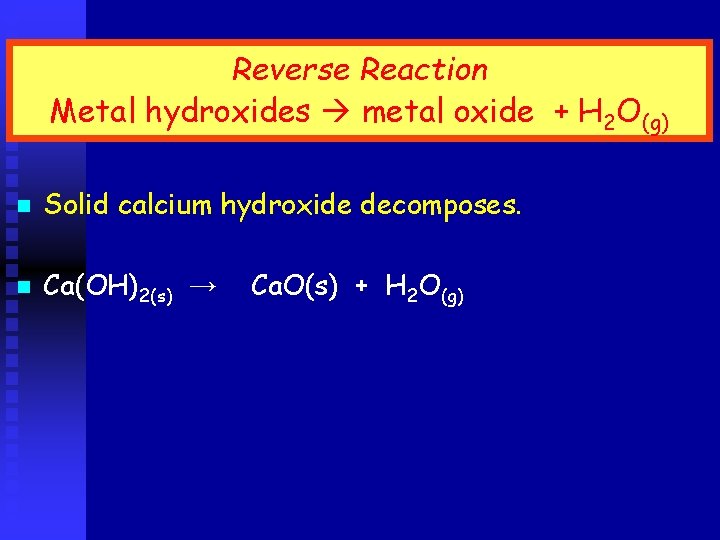
Reverse Reaction Metal hydroxides metal oxide + H 2 O(g) n Solid calcium hydroxide decomposes. n Ca(OH)2(s) → Ca. O(s) + H 2 O(g)

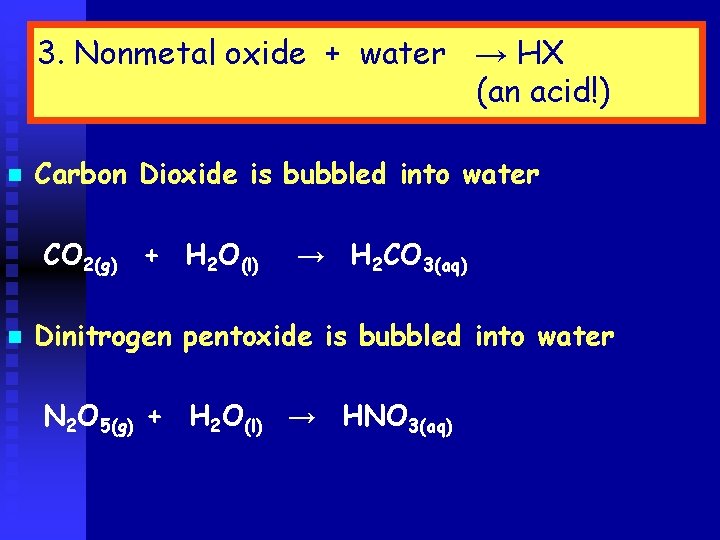
3. Nonmetal oxide + water → HX (an acid!) n Carbon Dioxide is bubbled into water CO 2(g) + H 2 O(l) n → H 2 CO 3(aq) Dinitrogen pentoxide is bubbled into water N 2 O 5(g) + H 2 O(l) → HNO 3(aq)

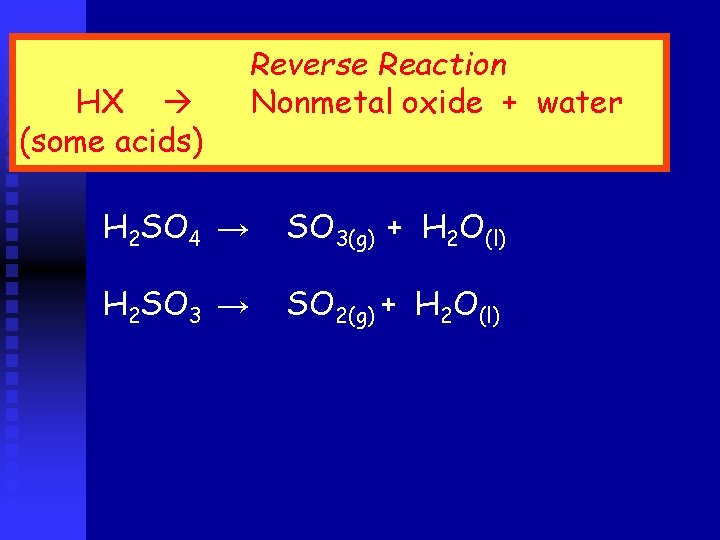
HX (some acids) Reverse Reaction Nonmetal oxide + water H 2 SO 4 → SO 3(g) + H 2 O(l) H 2 SO 3 → SO 2(g) + H 2 O(l)

4. Metal oxide + nonmetal oxide salt (ionic compound!) Solid sodium oxide is added to carbon dioxide Na 2 O + CO 2 Na 2 CO 3 Solid calcium oxide is added to sulfur trioxide Ca. O + SO 3 Ca. SO 4

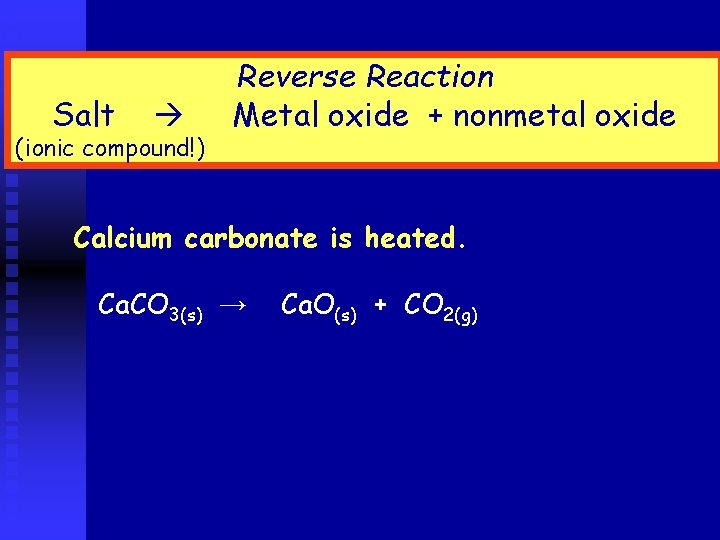
Salt (ionic compound!) Reverse Reaction Metal oxide + nonmetal oxide Calcium carbonate is heated. Ca. CO 3(s) → Ca. O(s) + CO 2(g)

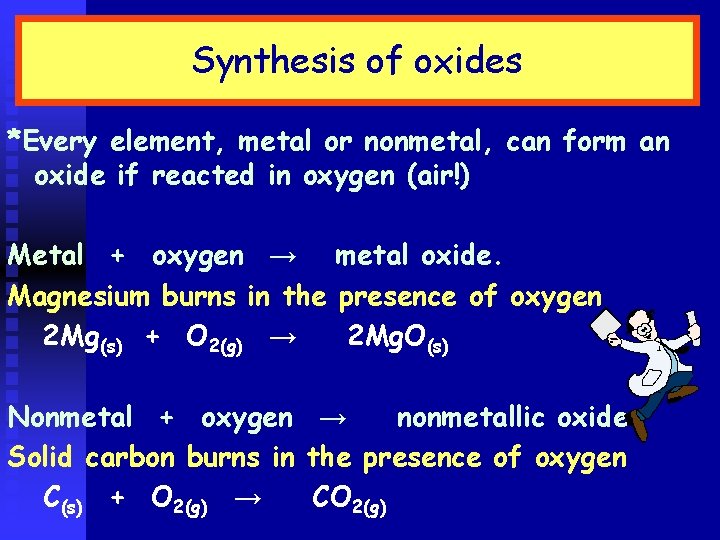
Synthesis of oxides *Every element, metal or nonmetal, can form an oxide if reacted in oxygen (air!) Metal + oxygen → metal oxide. Magnesium burns in the presence of oxygen 2 Mg(s) + O 2(g) → 2 Mg. O(s) Nonmetal + oxygen → nonmetallic oxide Solid carbon burns in the presence of oxygen C(s) + O 2(g) → CO 2(g)

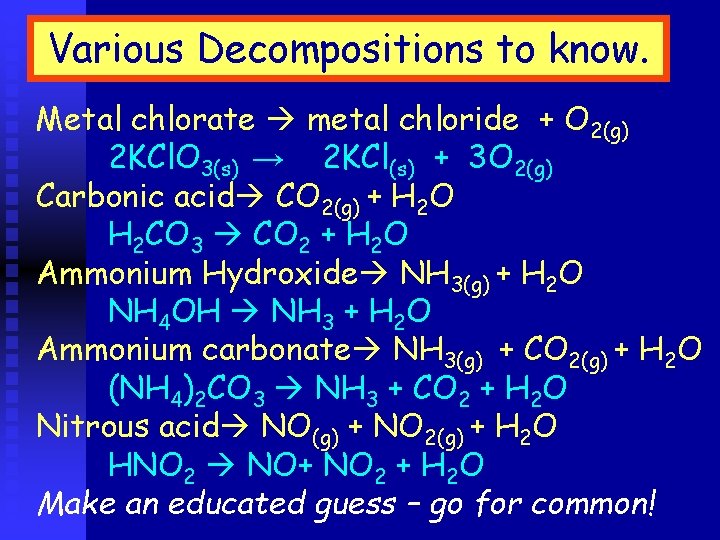
Various Decompositions to know. Metal chlorate metal chloride + O 2(g) 2 KCl. O 3(s) → 2 KCl(s) + 3 O 2(g) Carbonic acid CO 2(g) + H 2 O H 2 CO 3 CO 2 + H 2 O Ammonium Hydroxide NH 3(g) + H 2 O NH 4 OH NH 3 + H 2 O Ammonium carbonate NH 3(g) + CO 2(g) + H 2 O (NH 4)2 CO 3 NH 3 + CO 2 + H 2 O Nitrous acid NO(g) + NO 2(g) + H 2 O HNO 2 NO+ NO 2 + H 2 O Make an educated guess – go for common!

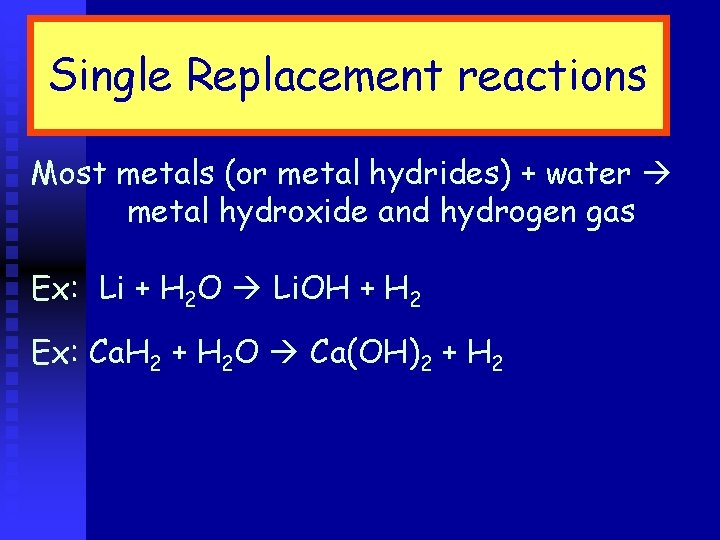
Single Replacement reactions Most metals (or metal hydrides) + water metal hydroxide and hydrogen gas Ex: Li + H 2 O Li. OH + H 2 Ex: Ca. H 2 + H 2 O Ca(OH)2 + H 2

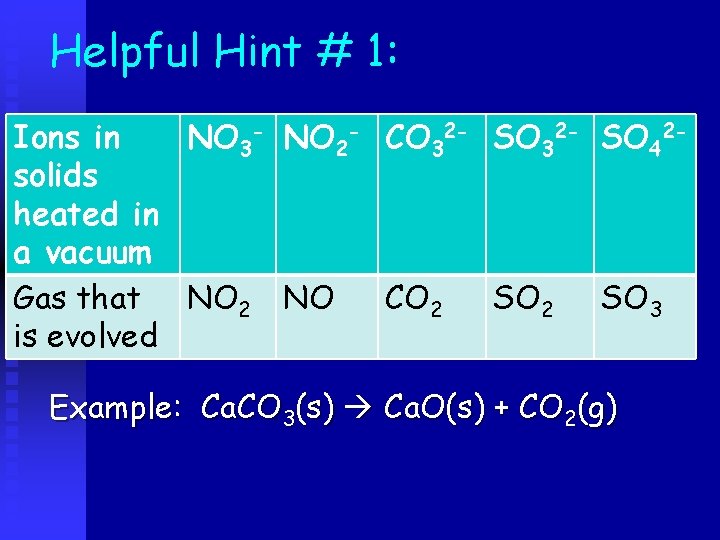
Helpful Hint # 1: Ions in NO 3 - NO 2 - CO 32 - SO 42 solids heated in a vacuum Gas that NO 2 NO CO 2 SO 3 is evolved Example: Ca. CO 3(s) Ca. O(s) + CO 2(g)

Helpful Hint 2: Certain salts react with acids to produce a gas. Common Gas H 2 S CO 2 SO 2 NH 3 Any salt sulfide(S-2) plus any acid form H 2 S + salt Ex: K 2 S + HCl KCl+H 2 S Any salt carbonate(CO 3 -2) plus any acid form HOH + CO 2 + salt Ex: HCl + Ca. CO 3 H 2 O + CO 2 + Ca. Cl 2 Any salt sulfite(SO 3 -2) plus any acid form HOH + SO 2 + salt Ex: Na 2 SO 3 + HCl H 2 O + SO 2 + Na. Cl Any ammonium salt (NH 4+) plus any strong hydroxide form NH 3 + HOH + salt Ex: NH 4 Cl + Na. OH NH 3 + HOH + Na. Cl