Some Questions to Consider Why are so few








![Lewis Dot Diagram Practice: Element Electron Configuration Li [He]2 s 1 Be [He]2 s Lewis Dot Diagram Practice: Element Electron Configuration Li [He]2 s 1 Be [He]2 s](https://slidetodoc.com/presentation_image_h2/57cd30750e0d05bc3aa8fad8a7f50f40/image-16.jpg)







- Slides: 28

Some Questions to Consider • Why are so few elements (such as Au, S, Ar, N, O, Ag) found free in Nature as atoms? • Why do atoms of different elements combine (react) to form compounds? • What is happening in this process? • How can we explain the tremendous number of compounds that are known today? • Many of the answers will be found in Chapter 6 (“Chemical Formulas and Bonding”), and they are the result of a ‘tug of war’ between elements. (Example)

Chapter 7: Chemical Formulas and Bonding PPowell 05

7 -1 Objectives • Describe the distinguishing characteristics of an ionic bond. • Describe some properties of ionic compounds. • Explain the “octet rule. ” • Draw Lewis dot diagrams to show the valence electrons of an atom. • Distinguish among anions, cations, and polyatomic ions. • Name binary ionic compounds. • Write the empirical formula for binary ionic compounds.

Ions: A Review • Cation: a positively charged ion. – Metals tend to form cations. (How? ) • Anion: a negatively charged ion. – Nonmetals tend to form anions. (How? ) • How do you think cations and anions will interact? OBJ: Describe the distinguishing characteristics of an ionic bond.

Ionic Compounds • Ionic bond: results from the attraction between a positive ion and a negative ion. – Most metals will form ionic bonds with most nonmetals. • Ionic compound: consists of cations and anions. – Electrically neutral; the total charges of the cations and anions must balance. OBJ: Describe the distinguishing characteristics of an ionic bond.

Video Clip: Ionic Bonds In Action • Reaction of magnesium and oxygen releases light energy. • Product (Mg. O) is more stable than the reactants alone. OBJ: Describe the distinguishing characteristics of an ionic bond.

Why is magnesium oxide more stable than elemental magnesium and oxygen? • Compare electron configurations of the atoms to their ions: Ø Mg: [Ne]3 s 2 Ø Mg 2+ + 2 e • Mg 2+ = [Ne]2+ Ø O: [He]2 s 22 p 4 Ø O + 2 e- O 2 • O 2 -: [He]2 s 22 p 6, or [Ne]2 - • Noble Gas electron configurations are more stable! 7 -1 A: Describe the distinguishing characteristics of an ionic bond.

The Octet Rule • Atoms tend to gain, lose, or share electrons in order to get a full set of valence electrons. • Most atoms have eight valence electrons in a full set. – Exceptions: H, He (Why? ) • The representative elements act like “noble gas impersonators. ” OBJ: Explain the octet rule.

The Octet Rule • Metals will lose e- to “impersonate” a noble gas. – Metals form cations. • Nonmetals will gain e- to “impersonate” a noble gas. – Nonmetals form anions. OBJ: Explain the octet rule.

The Role of Valence Electrons • Note that the valence electrons were involved in these changes, NOT the core electrons. – Why? (Which orbitals & electrons are encountered first when two atoms interact? ) • Chemists focus on the valence electrons (s and p outer electrons) to understand the chemistry of atoms. • To aid us, we use shorthand diagrams, called Lewis Dot Diagrams, where dots represent the valence electrons around an atom.

LEWIS DOT DIAGRAMS

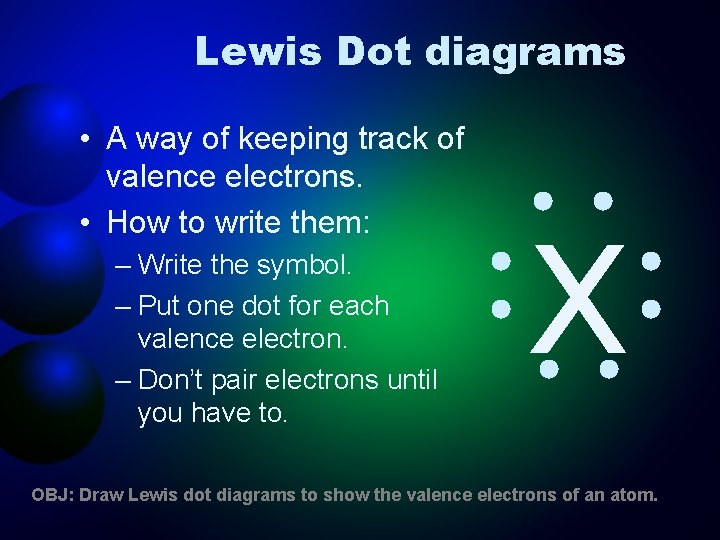
Lewis Dot diagrams • A way of keeping track of valence electrons. • How to write them: – Write the symbol. – Put one dot for each valence electron. – Don’t pair electrons until you have to. X OBJ: Draw Lewis dot diagrams to show the valence electrons of an atom.

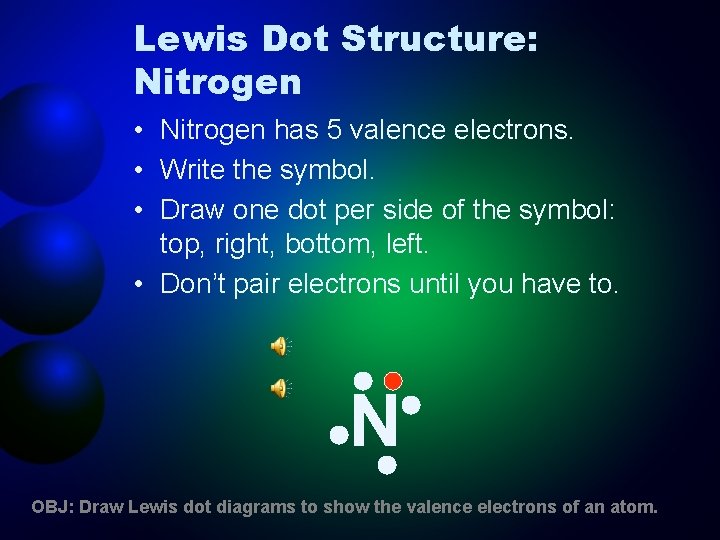
Lewis Dot Structure: Nitrogen • Nitrogen has 5 valence electrons. • Write the symbol. • Draw one dot per side of the symbol: top, right, bottom, left. • Don’t pair electrons until you have to. N OBJ: Draw Lewis dot diagrams to show the valence electrons of an atom.

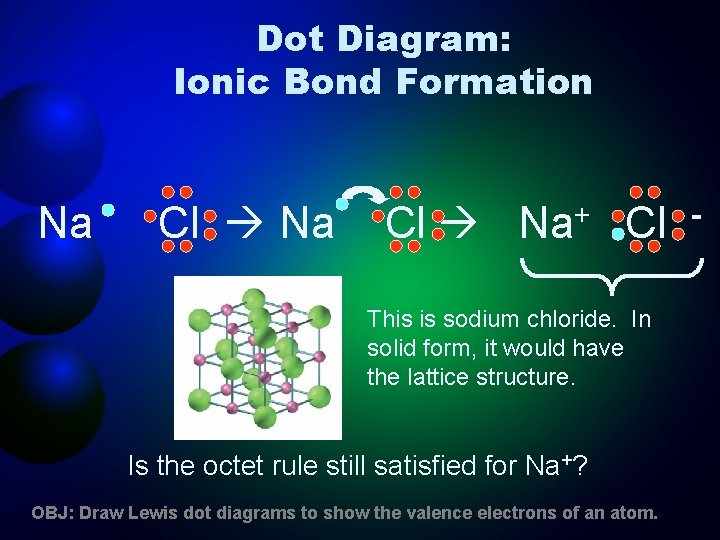
Dot Diagram: Ionic Bond Formation Na Cl Na+ Cl This is sodium chloride. In solid form, it would have the lattice structure. Is the octet rule still satisfied for Na+? OBJ: Draw Lewis dot diagrams to show the valence electrons of an atom. -

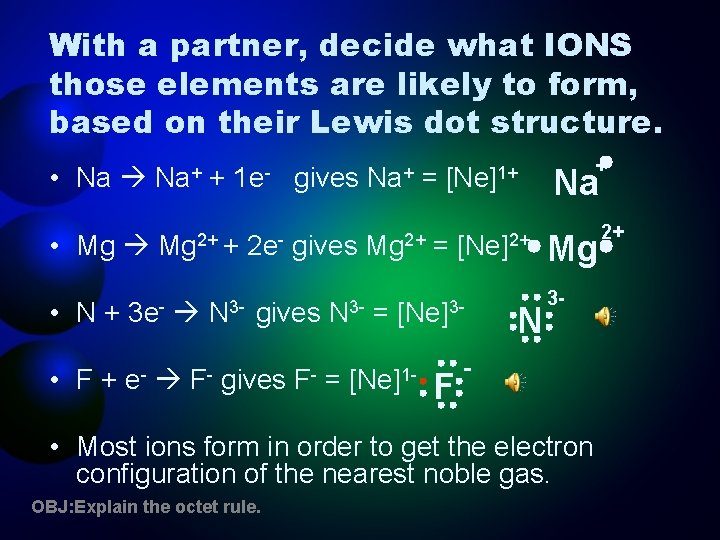
With a partner, decide what IONS those elements are likely to form, based on their Lewis dot structure. • Na Na+ + 1 e- gives Na+ = [Ne]1+ • Mg 2+ + • N+ 3 e- • F+ e- N 3 - F- 2 e- gives F- N 3= Mg 2+ = = [Ne]2+ [Ne]3 - [Ne]1 - F N + Na Mg 3 - - • Most ions form in order to get the electron configuration of the nearest noble gas. OBJ: Explain the octet rule. 2+
![Lewis Dot Diagram Practice Element Electron Configuration Li He2 s 1 Be He2 s Lewis Dot Diagram Practice: Element Electron Configuration Li [He]2 s 1 Be [He]2 s](https://slidetodoc.com/presentation_image_h2/57cd30750e0d05bc3aa8fad8a7f50f40/image-16.jpg)
Lewis Dot Diagram Practice: Element Electron Configuration Li [He]2 s 1 Be [He]2 s 2 B [He]2 s 22 p 1 C [He]2 s 22 p 2 N [He]2 s 22 p 3 O [He]2 s 22 p 4 F [He]2 s 22 p 5 Ne [He]2 s 22 p 6 Al [Ne]3 s 23 p 1 P [Ne]3 s 23 p 3 Lewis Dot Diagram

Important Note • When an anion is formed, the electron(s) come(s) from some other atom. • When a cation is formed, the original atom has donated its electron(s) to some other atom. • In other words, the previous slide only showed you only half the story. OBJ: Describe the distinguishing characteristics of an ionic bond.

What about Argon? • Ar is a noble gas. • Ar has an octet. • Ar is INERT, or unreactive. Ar OBJ: Explain the octet rule.

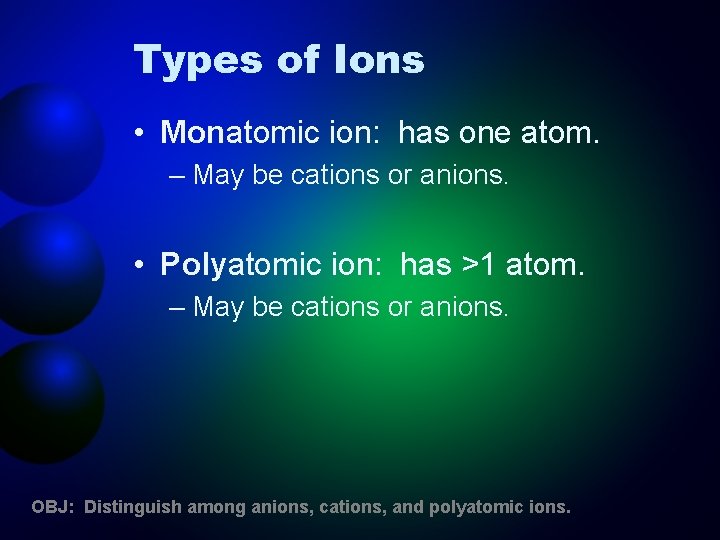
Types of Ions • Monatomic ion: has one atom. – May be cations or anions. • Polyatomic ion: has >1 atom. – May be cations or anions. OBJ: Distinguish among anions, cations, and polyatomic ions.

Monatomic Cations • See p. 231 Figure 7 -10 Monatomic Anions • See p. 232 Figure 7 -11 Polyatomic Ions • Can you find them in Figures 7 -10 and 7 -11? OBJ: Distinguish among anions, cations, and polyatomic ions.

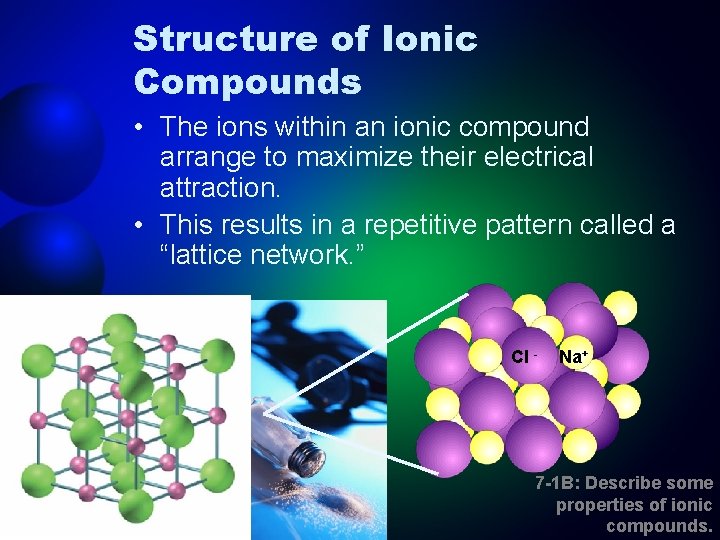
Structure of Ionic Compounds • The ions within an ionic compound arrange to maximize their electrical attraction. • This results in a repetitive pattern called a “lattice network. ” Cl - Na+ 7 -1 B: Describe some properties of ionic compounds.

Properties of Ionic Compounds • High bond strength means high melting points. • Often dissolve in water (soluble). + • + + - + - + • + - + - + – Ions in solution move freely and allow the solution to conduct electricity. Ionic compounds are not good conductors as a solid, but they are when melted. Ionic compounds are brittle. 7 -1 B: Describe some properties of ionic compounds.

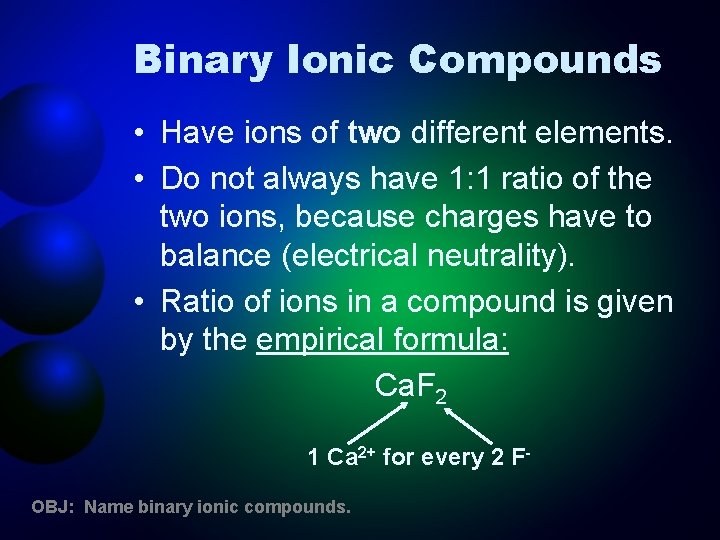
Binary Ionic Compounds • Have ions of two different elements. • Do not always have 1: 1 ratio of the two ions, because charges have to balance (electrical neutrality). • Ratio of ions in a compound is given by the empirical formula: Ca. F 2 1 Ca 2+ for every 2 FOBJ: Name binary ionic compounds.

Naming Binary Ionic Compounds Ca. F 2 • Name the cation, then the anion. • Change the ending of the anion to “ide. ” Calcium Fluoride Try naming these: a) Potassium Chloride b) Iron Oxide a) KCl b) Fe. O c) Zinc Fluoride c) Zn. F 2 d) Ammonium Chloride d) NH 4 Cl OBJ: Name binary ionic compounds.

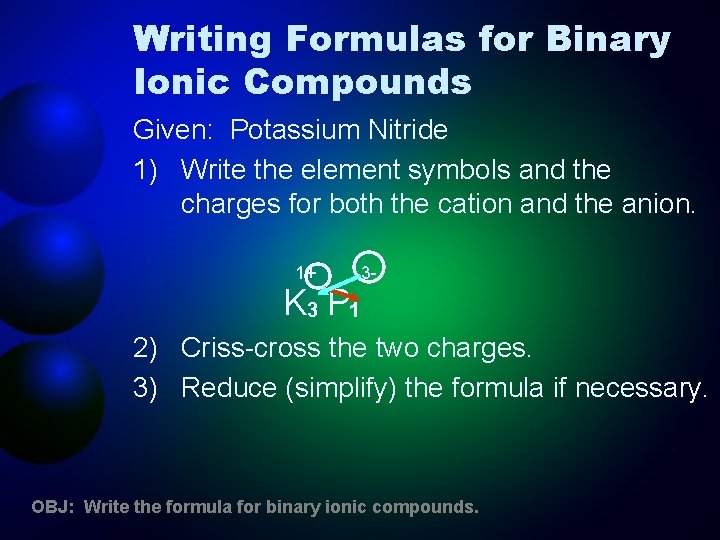
Writing Formulas for Binary Ionic Compounds Given: Potassium Nitride 1) Write the element symbols and the charges for both the cation and the anion. 1+ K 3 P 1 3 - 2) Criss-cross the two charges. 3) Reduce (simplify) the formula if necessary. OBJ: Write the formula for binary ionic compounds.

7 -1 Practice Problems • Do problems 1 -5 with a partner. • Do problems 6 -10 on your own. OBJ: Write the formula for binary ionic compounds.

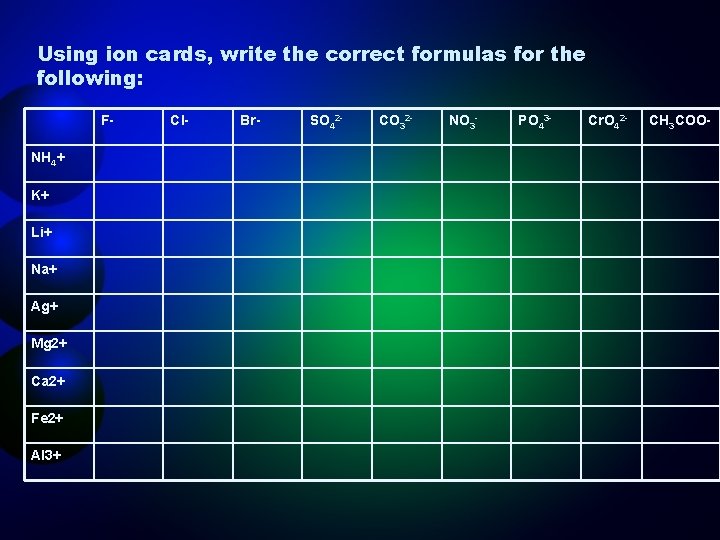
Using ion cards, write the correct formulas for the following: FNH 4+ K+ Li+ Na+ Ag+ Mg 2+ Ca 2+ Fe 2+ Al 3+ Cl- Br- SO 42 - CO 32 - NO 3 - PO 43 - Cr. O 42 - CH 3 COO-

Can you now meet the objectives for 7 -1? • Describe the distinguishing characteristics of an ionic bond. • Describe some properties of ionic compounds. • Explain the “octet rule. ” • Draw Lewis dot diagrams to show the valence electrons of an atom. • Distinguish among anions, cations, and polyatomic ions. • Name binary ionic comounds. • Write the formula for binary ionic compounds.