Some basic concepts of organic chemistry By Mayank

Some basic concepts of organic chemistry By Mayank Kumar
Factors affecting electron availability: • Inductive effect: • In a covalent single bond between unlike atoms, the electron pair forming a sigma bond is never shared equally between the two atoms, it tends to be attracted a little more towards the more electronegative atom of the two. For example in alkyl chloride the electron density is attracted more towards chloride than carbon due to chloride being more electronegative. • This difference in electronegativity causes polarization in covalent bonds and this polarization is responsible for predicting acidic and basic behavior in many organic compounds.
• The electron distribution in sigma bond is known as inductive effet. • It is a permanent effect and applies to the 3 RD carbon in the chain. • Dipole moments of molecules can also be predicted with the help of this effect • Inductive effect is of two types: -I effect & +I effect In –I effect a electron withdrawing element or the element with higher electronegativity than carbon is attached to the carbon atom. The carbon atom to which this group is directly attached is called alpha carbon, the next carbon atom is beta and the third carbon is gamma. Inductive effect operated till gamma carbon only. This effect increases the acidity of hydrogen attached to carbon because it bond between carbon and hydrogen becomes weak making the hydrogen removal easy.
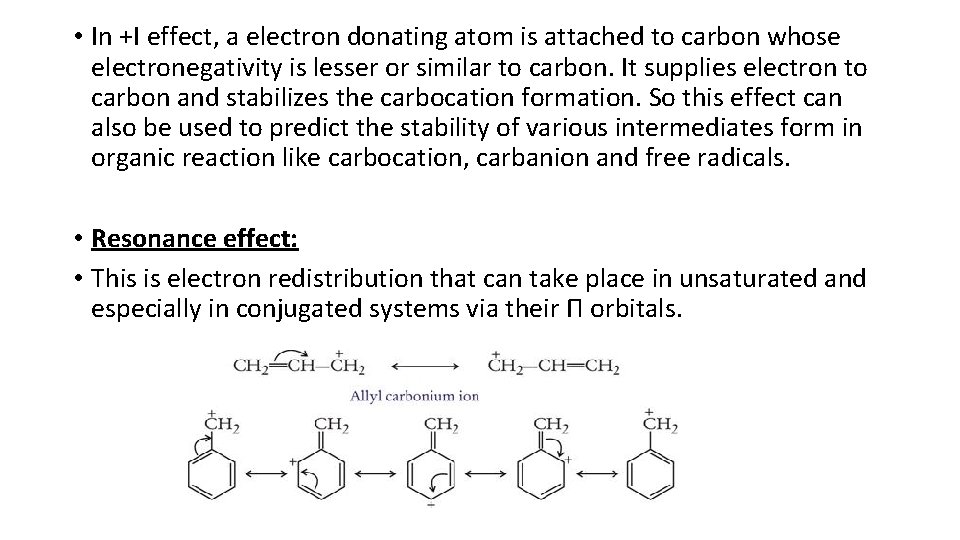
• In +I effect, a electron donating atom is attached to carbon whose electronegativity is lesser or similar to carbon. It supplies electron to carbon and stabilizes the carbocation formation. So this effect can also be used to predict the stability of various intermediates form in organic reaction like carbocation, carbanion and free radicals. • Resonance effect: • This is electron redistribution that can take place in unsaturated and especially in conjugated systems via their Π orbitals.
• When double bond or lone pairs are in conjugation with any charge then movement of electrons from higher density to lower electron density takes place, that is called resonance. In the above diagram you can see that a structure having resonance can be represented in more than one way. The actual structure is the hybrid of all canonical forms and the most stable canonical form among all the structures best define the properties of that compound. • When resonance and inductive, both effects are operated in the same compound then resonance is given preference over inductive to predict the behavior of that compound. • Like inductive resonance can also be of two types. • Resonance provides stability to the charged molecule by delocalization of charge when it is present in conjugation.
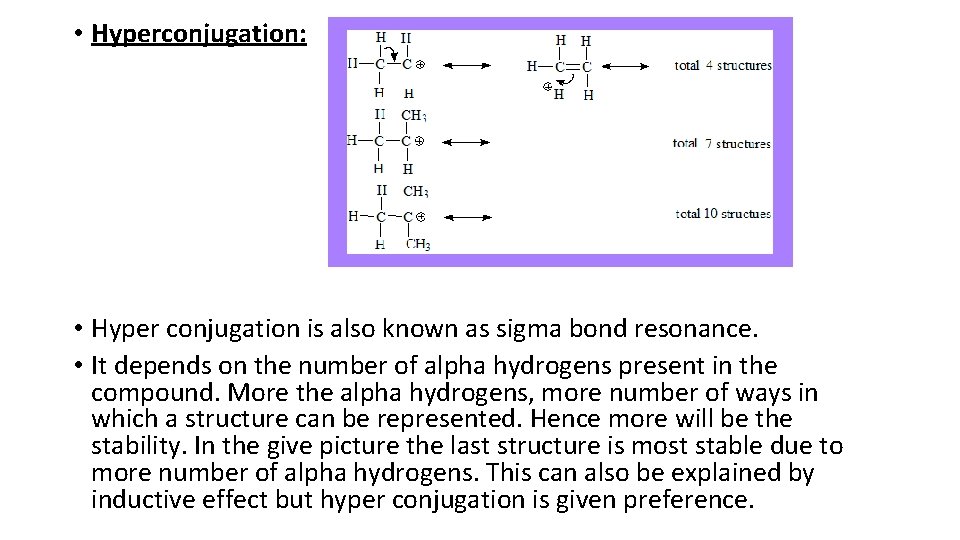
• Hyperconjugation: • Hyper conjugation is also known as sigma bond resonance. • It depends on the number of alpha hydrogens present in the compound. More the alpha hydrogens, more number of ways in which a structure can be represented. Hence more will be the stability. In the give picture the last structure is most stable due to more number of alpha hydrogens. This can also be explained by inductive effect but hyper conjugation is given preference.
- Slides: 6