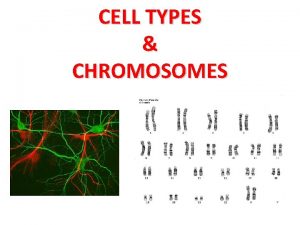
Somatic evolution and cancer Natalia Komarova University of
















































- Slides: 146

Somatic evolution and cancer Natalia Komarova (University of California - Irvine)

Plan • • Introduction: The concept of somatic evolution Methodology: Stochastic processes on selection-mutation networks Two particular problems: 1. Stem cells, initiation of cancer and optimal tissue architecture (with L. Wang and P. Cheng) 2. Drug therapy and generation of resistance: neutral evolution inside a tumor (with D. Wodarz)

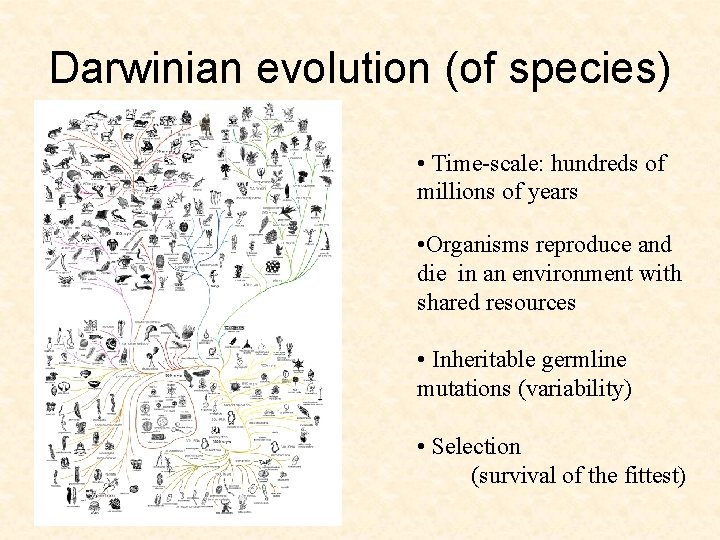
Darwinian evolution (of species) • Time-scale: hundreds of millions of years • Organisms reproduce and die in an environment with shared resources

Darwinian evolution (of species) • Time-scale: hundreds of millions of years • Organisms reproduce and die in an environment with shared resources • Inheritable germline mutations (variability) • Selection (survival of the fittest)

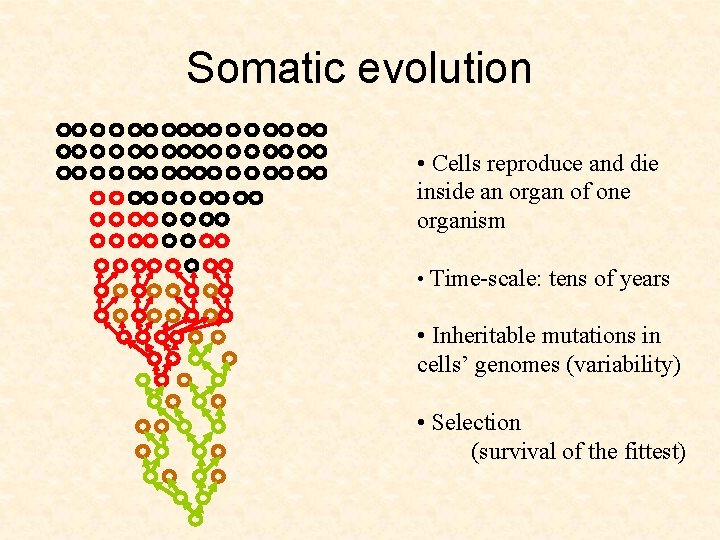
Somatic evolution • Cells reproduce and die inside an organ of one organism • Time-scale: tens of years

Somatic evolution • Cells reproduce and die inside an organ of one organism • Time-scale: tens of years • Inheritable mutations in cells’ genomes (variability) • Selection (survival of the fittest)

Cancer as somatic evolution • Cells in a multicellular organism have evolved to cooperate and perform their respective functions for the good of the whole organism

Cancer as somatic evolution • Cells in a multicellular organism have evolved to cooperate and perform their respective functions for the good of the whole organism • A mutant cell that “refuses” to co-operate may have a selective advantage

Cancer as somatic evolution • Cells in a multicellular organism have evolved to cooperate and perform their respective functions for the good of the whole organism • A mutant cell that “refuses” to co-operate may have a selective advantage • The offspring of such a cell may spread

Cancer as somatic evolution • Cells in a multicellular organism have evolved to cooperate and perform their respective functions for the good of the whole organism • A mutant cell that “refuses” to co-operate may have a selective advantage • The offspring of such a cell may spread • This is a beginning of cancer

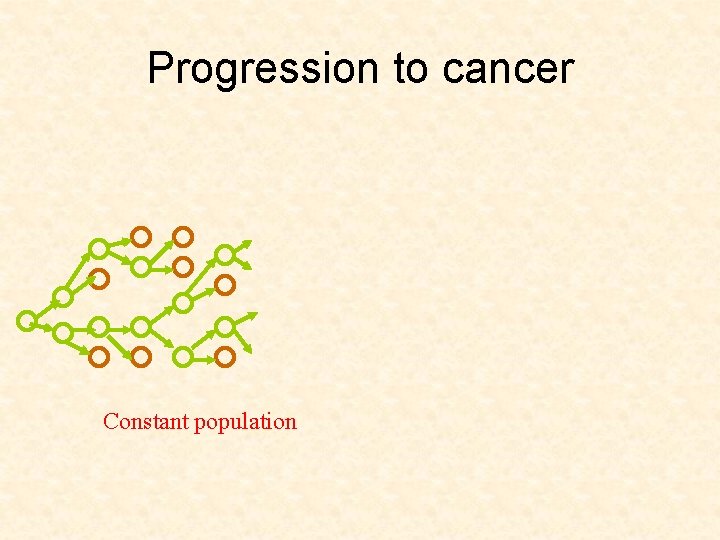
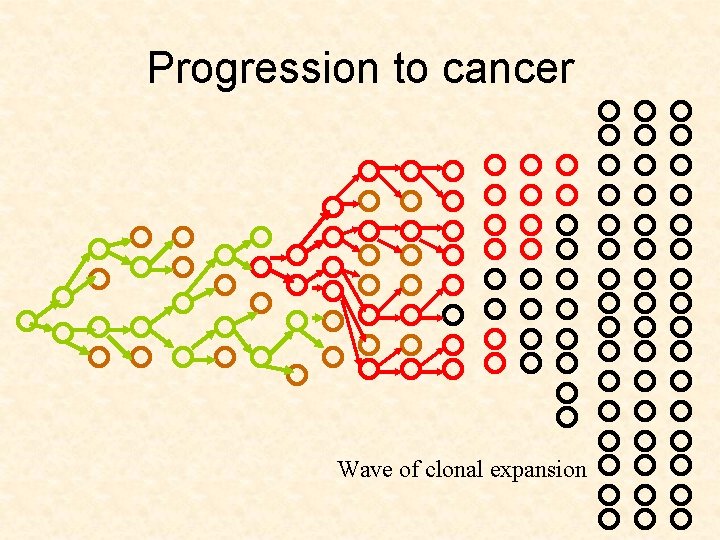
Progression to cancer

Progression to cancer Constant population

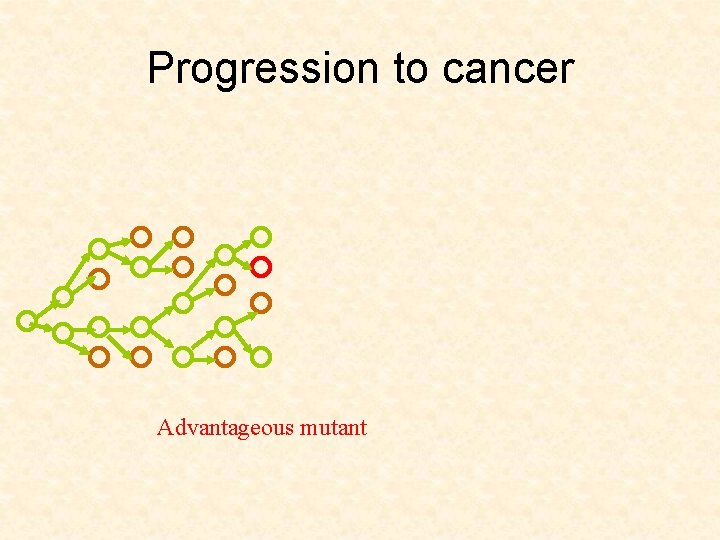
Progression to cancer Advantageous mutant

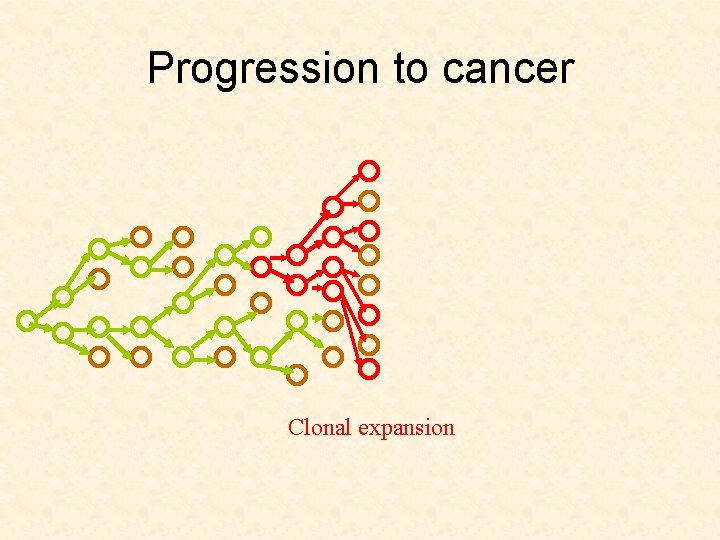
Progression to cancer Clonal expansion

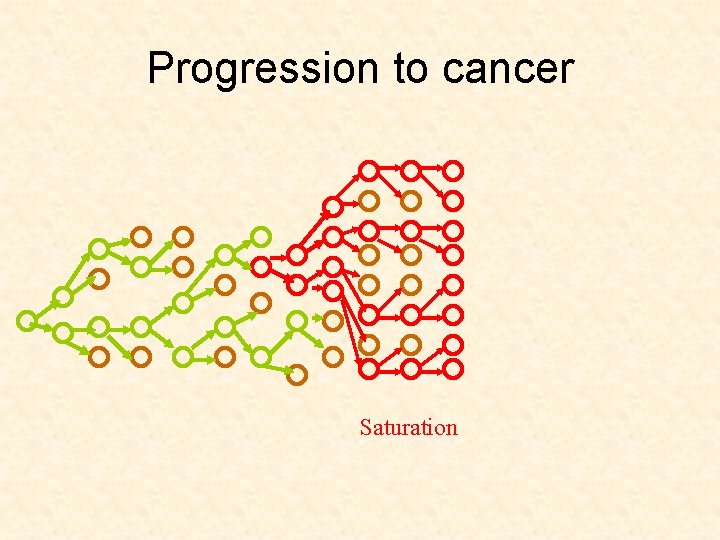
Progression to cancer Saturation

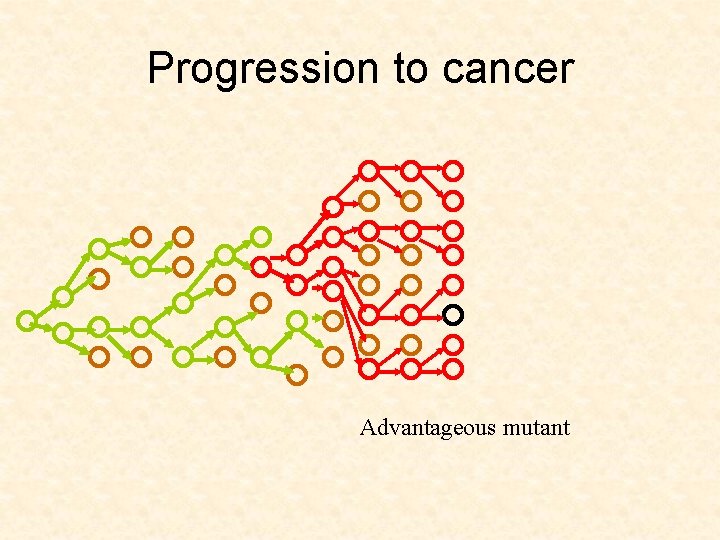
Progression to cancer Advantageous mutant

Progression to cancer Wave of clonal expansion

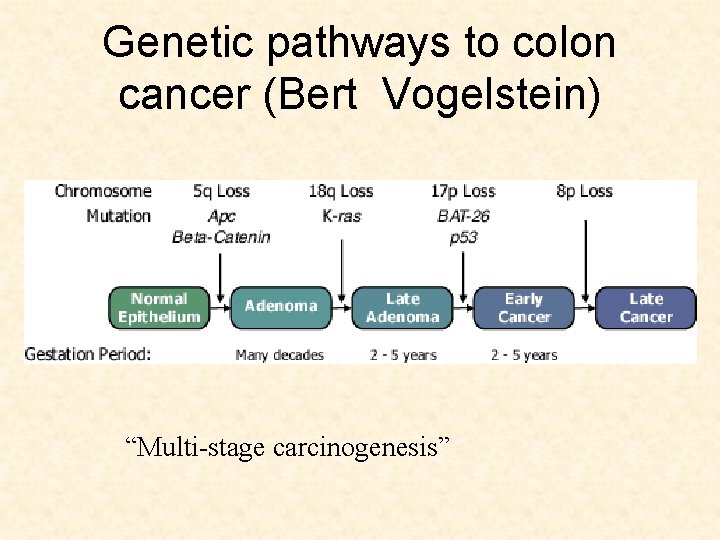
Genetic pathways to colon cancer (Bert Vogelstein) “Multi-stage carcinogenesis”

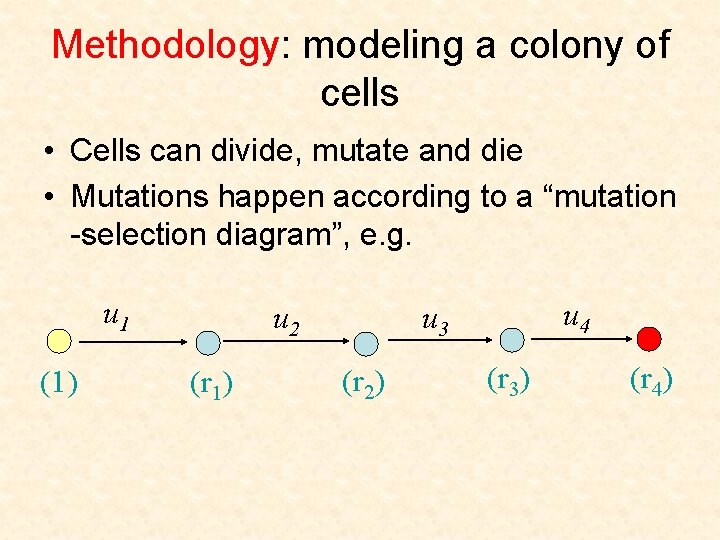
Methodology: modeling a colony of cells • Cells can divide, mutate and die

Methodology: modeling a colony of cells • Cells can divide, mutate and die • Mutations happen according to a “mutation -selection diagram”, e. g. u 1 (1) u 2 (r 1) u 4 u 3 (r 2) (r 3) (r 4)

Mutation-selection network (r 2) u 8 (r 3) u 8 (1) u 2 (r 1) u 5 u 2 u 5 (r 4) (r 5) (r 6) u 8 (r 7)

Stochastic dynamics on a selectionmutation network

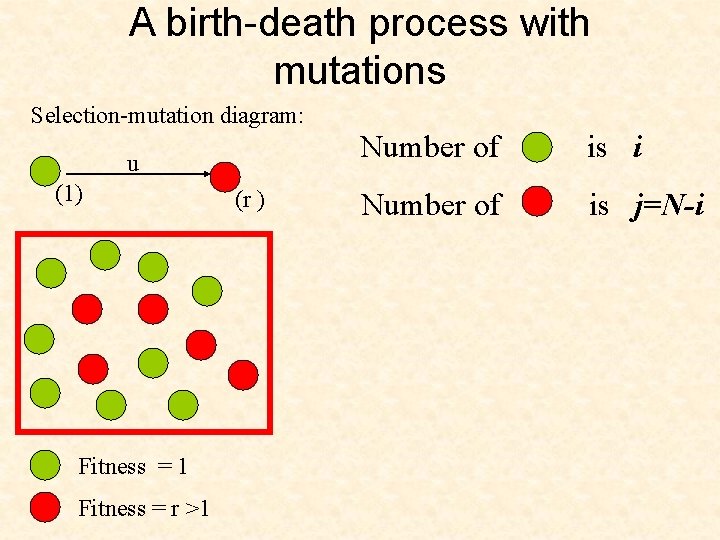
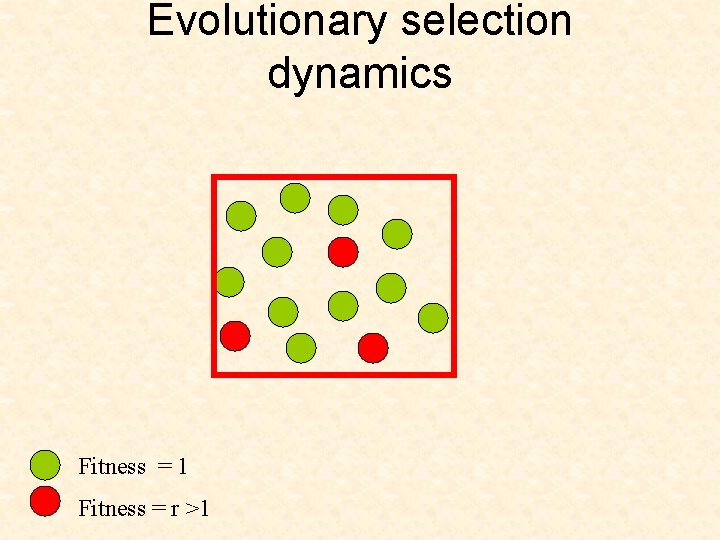
A birth-death process with mutations Selection-mutation diagram: u (1) Fitness = 1 Fitness = r >1 (r ) Number of is i Number of is j=N-i

Evolutionary selection dynamics Fitness = 1 Fitness = r >1

Evolutionary selection dynamics Fitness = 1 Fitness = r >1

Evolutionary selection dynamics Fitness = 1 Fitness = r >1

Evolutionary selection dynamics Fitness = 1 Fitness = r >1

Evolutionary selection dynamics Fitness = 1 Fitness = r >1

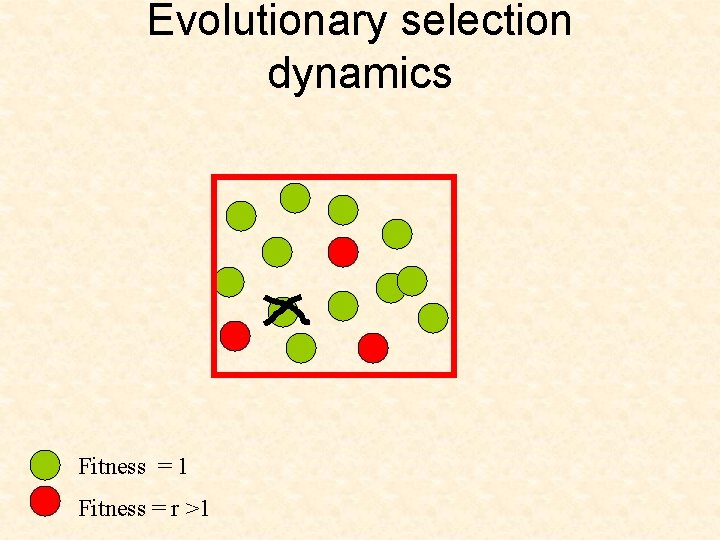
Evolutionary selection dynamics Start from only one cell of the second type. Suppress further mutations. What is the chance that it will take over? Fitness = 1 Fitness = r >1

Evolutionary selection dynamics Start from only one cell of the second type. What is the chance that it will take over? Fitness = 1 Fitness = r >1 If If r=1 then = 1/N r<1 then < 1/N r>1 then > 1/N r then =1

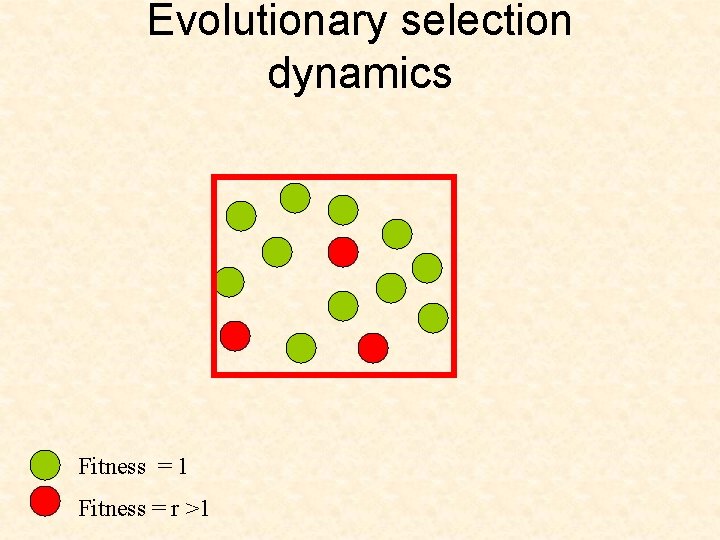
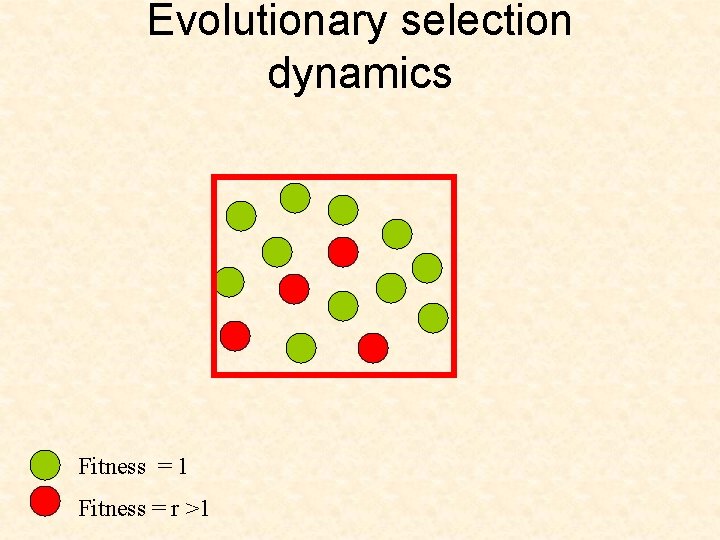
Evolutionary selection dynamics Start from zero cell of the second type. What is the expected time until the second type takes over? Fitness = 1 Fitness = r >1

Evolutionary selection dynamics Start from zero cell of the second type. What is the expected time until the second type takes over? In the case of rare mutations, we can show that Fitness = 1 Fitness = r >1

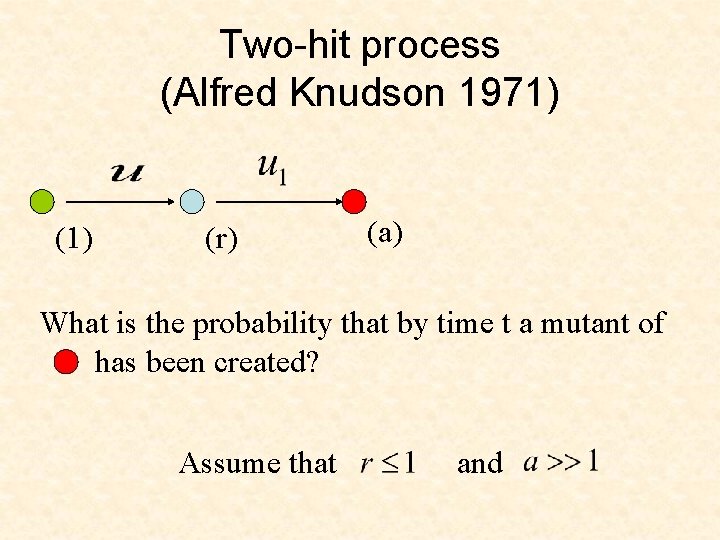
Two-hit process (Alfred Knudson 1971) (r) (a) What is the probability that by time t a mutant of has been created? Assume that and

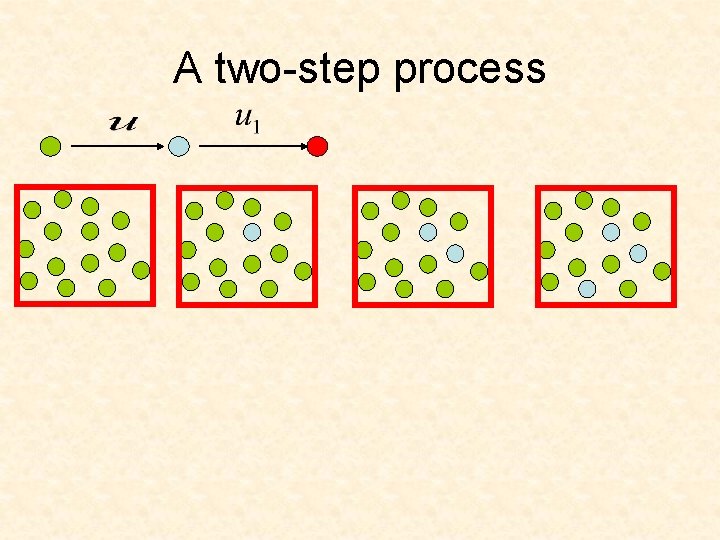
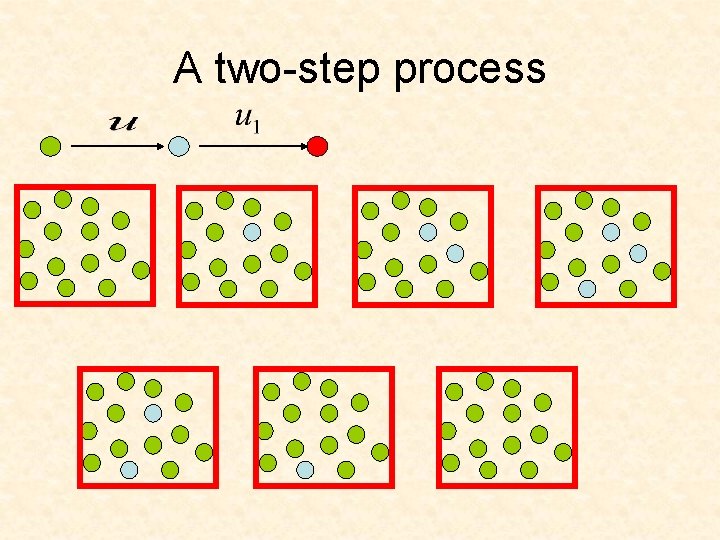
A two-step process

A two-step process

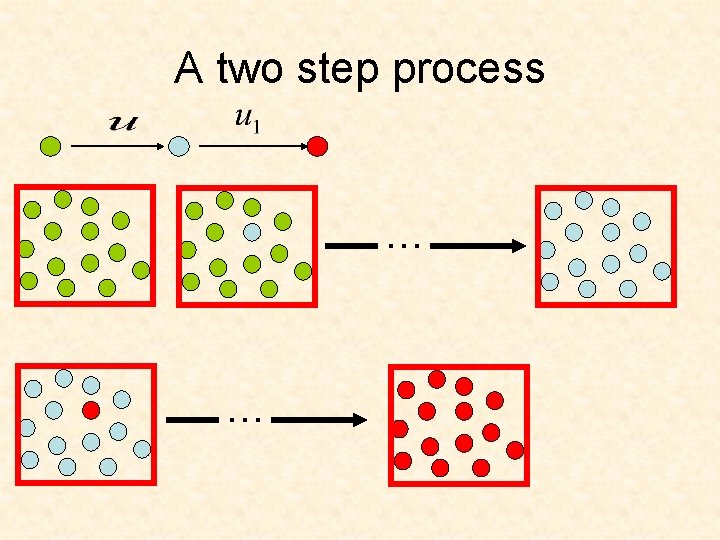
A two step process … …

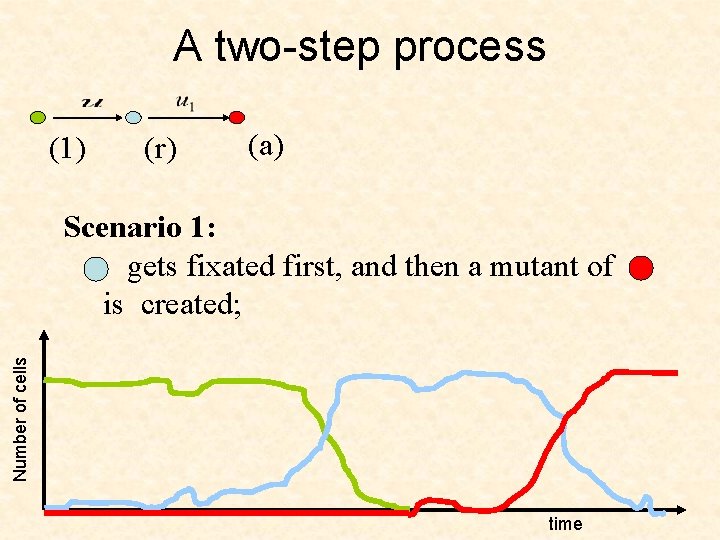
A two-step process (1) (r) (a) Number of cells Scenario 1: gets fixated first, and then a mutant of is created; time

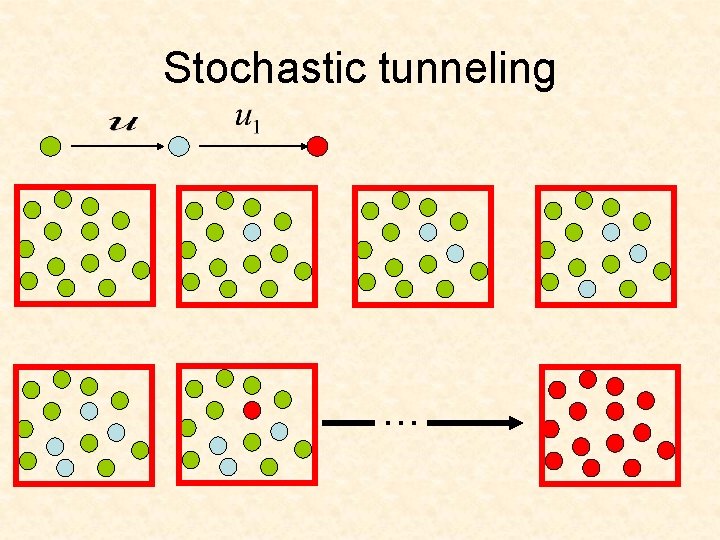
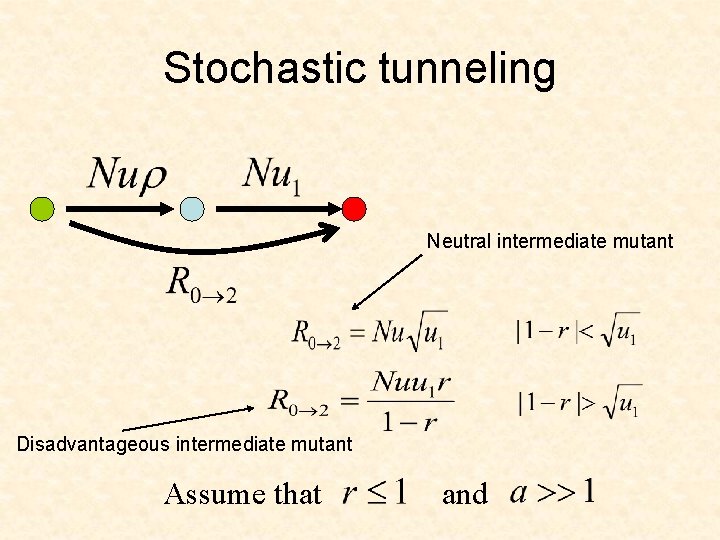
Stochastic tunneling …

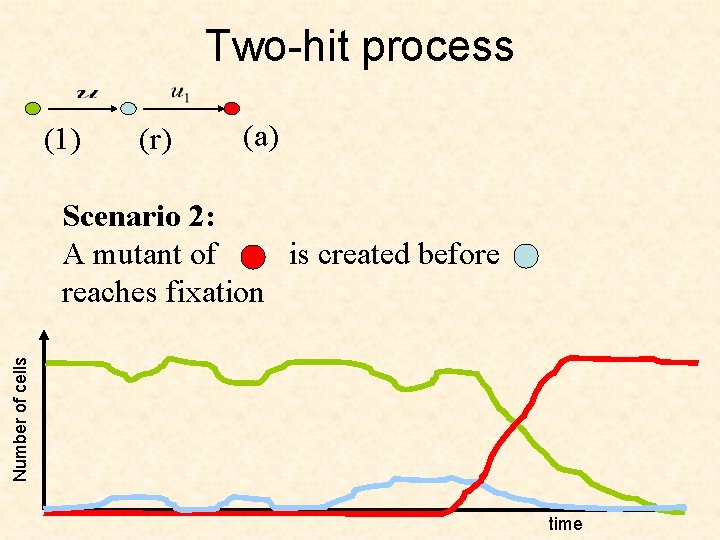
Two-hit process (1) (r) (a) Number of cells Scenario 2: A mutant of is created before reaches fixation time

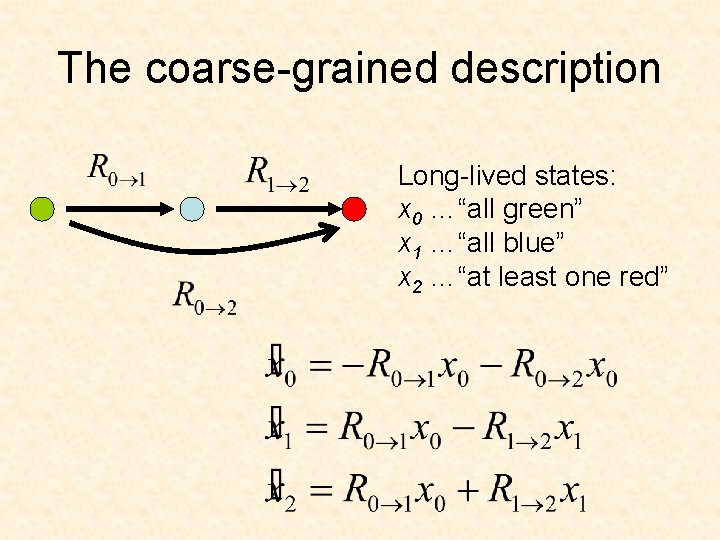
The coarse-grained description Long-lived states: x 0 …“all green” x 1 …“all blue” x 2 …“at least one red”

Stochastic tunneling Neutral intermediate mutant Disadvantageous intermediate mutant Assume that and

Stem cells, initiation of cancer and optimal tissue architecture

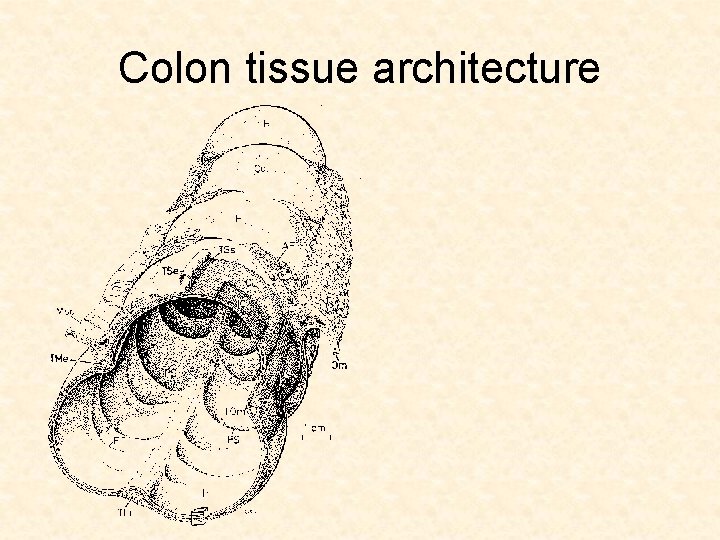
Colon tissue architecture

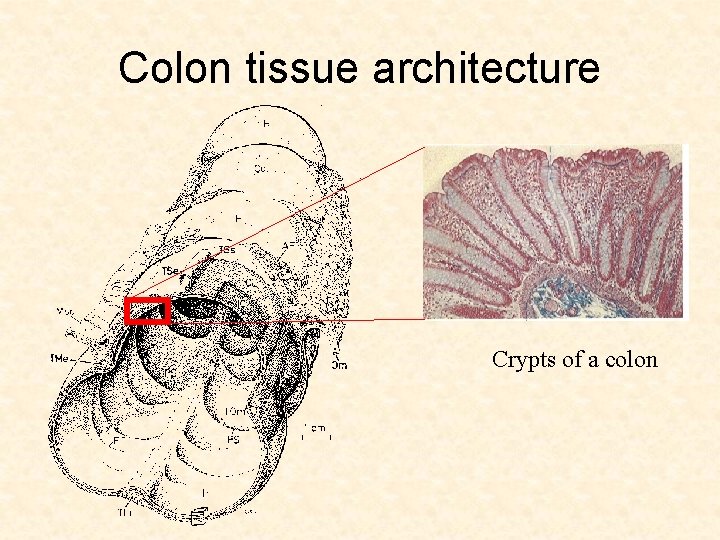
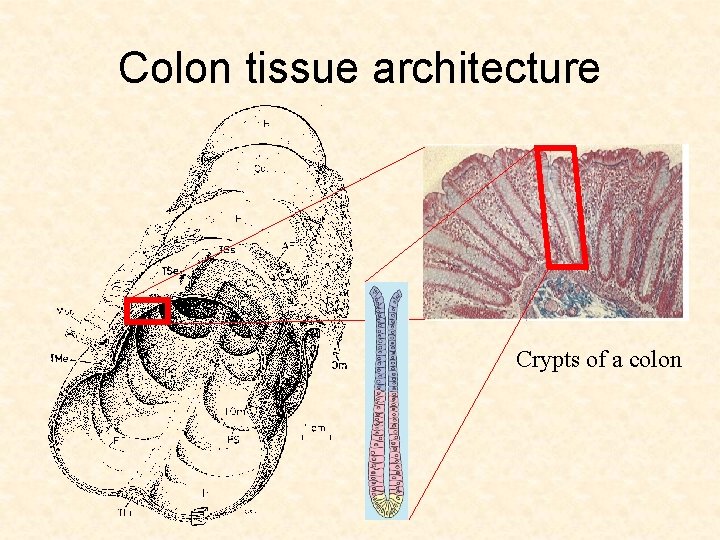
Colon tissue architecture Crypts of a colon

Colon tissue architecture Crypts of a colon

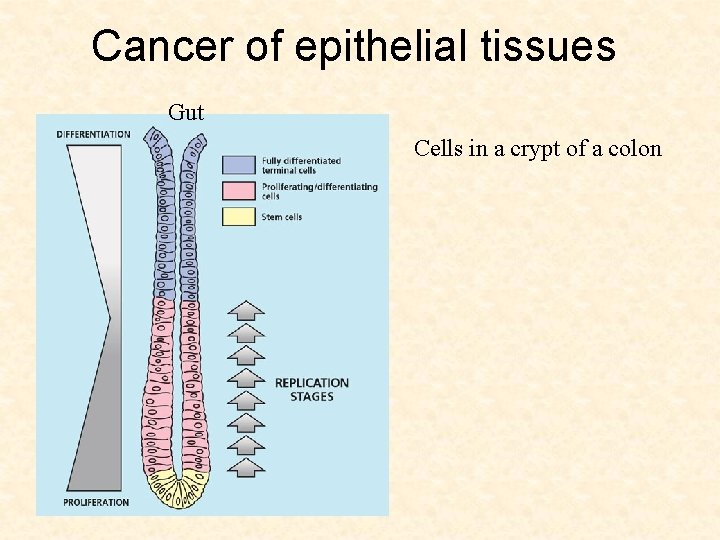
Cancer of epithelial tissues Gut Cells in a crypt of a colon

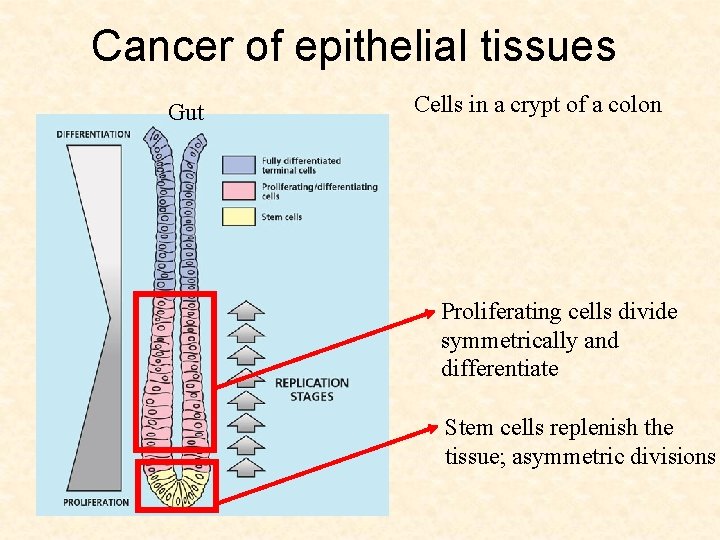
Cancer of epithelial tissues Gut Cells in a crypt of a colon Stem cells replenish the tissue; asymmetric divisions

Cancer of epithelial tissues Gut Cells in a crypt of a colon Proliferating cells divide symmetrically and differentiate Stem cells replenish the tissue; asymmetric divisions

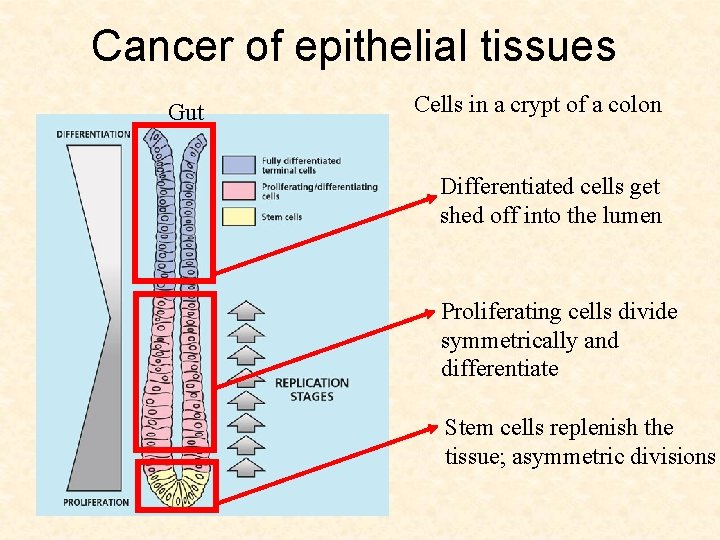
Cancer of epithelial tissues Gut Cells in a crypt of a colon Differentiated cells get shed off into the lumen Proliferating cells divide symmetrically and differentiate Stem cells replenish the tissue; asymmetric divisions

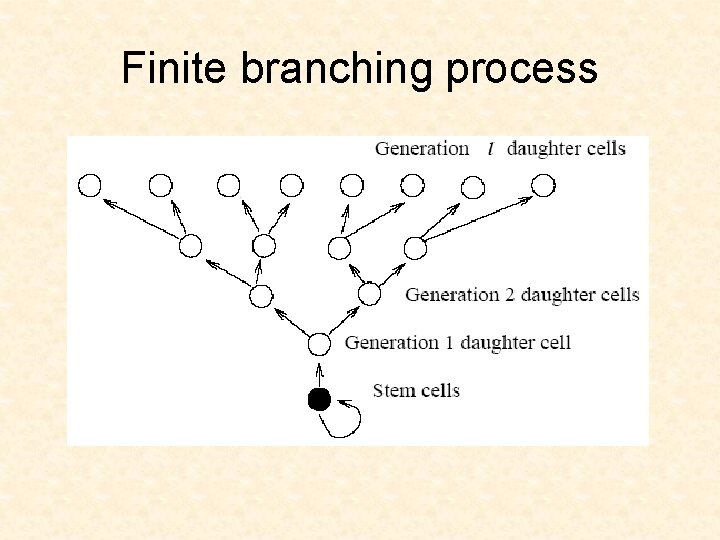
Finite branching process

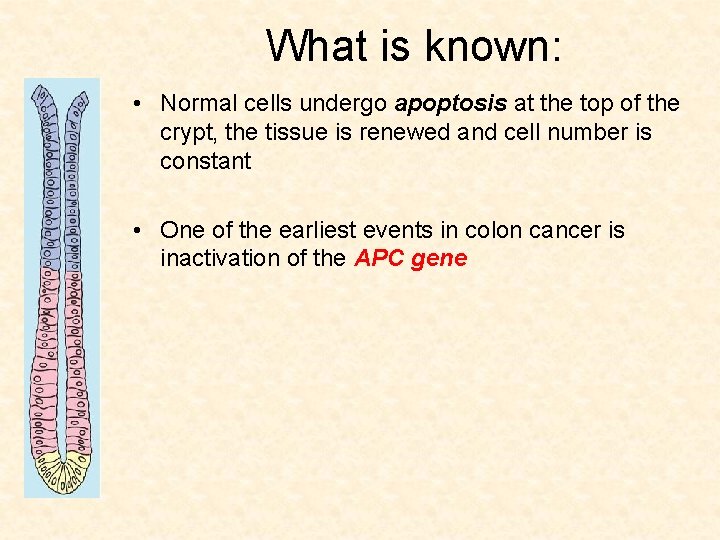
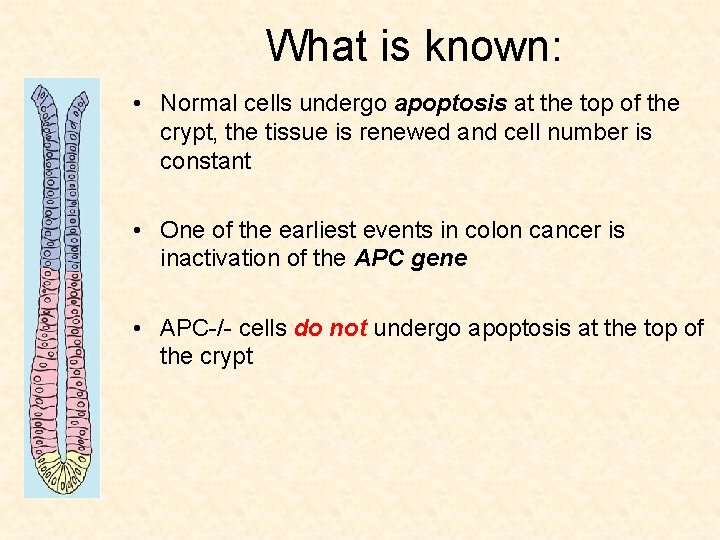
What is known: • Normal cells undergo apoptosis at the top of the crypt, the tissue is renewed and cell number is constant

What is known: • Normal cells undergo apoptosis at the top of the crypt, the tissue is renewed and cell number is constant • One of the earliest events in colon cancer is inactivation of the APC gene

What is known: • Normal cells undergo apoptosis at the top of the crypt, the tissue is renewed and cell number is constant • One of the earliest events in colon cancer is inactivation of the APC gene • APC-/- cells do not undergo apoptosis at the top of the crypt

What is NOT known: ? • What is the cellular origin of cancer? • Which cells harbor the first dangerous mutaton? Are the stem cells the ones in danger? ? ? • Which compartment must be targeted by drugs?

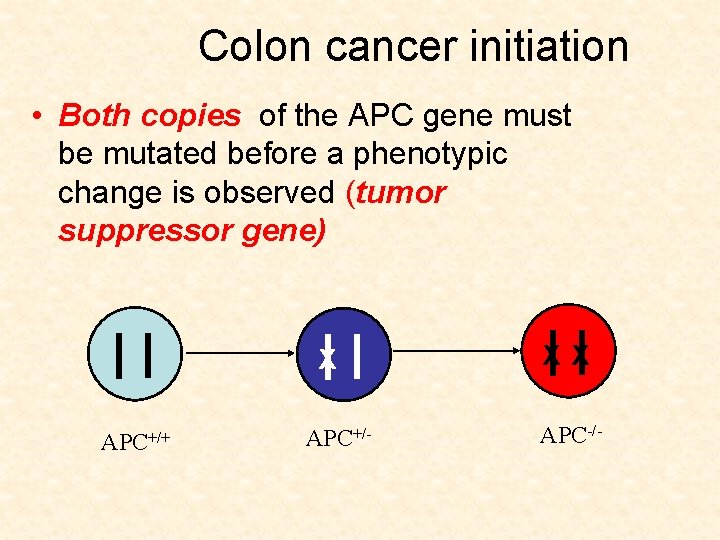
Colon cancer initiation • Both copies of the APC gene must be mutated before a phenotypic change is observed (tumor suppressor gene) X APC+/+ APC+/- XX APC-/-

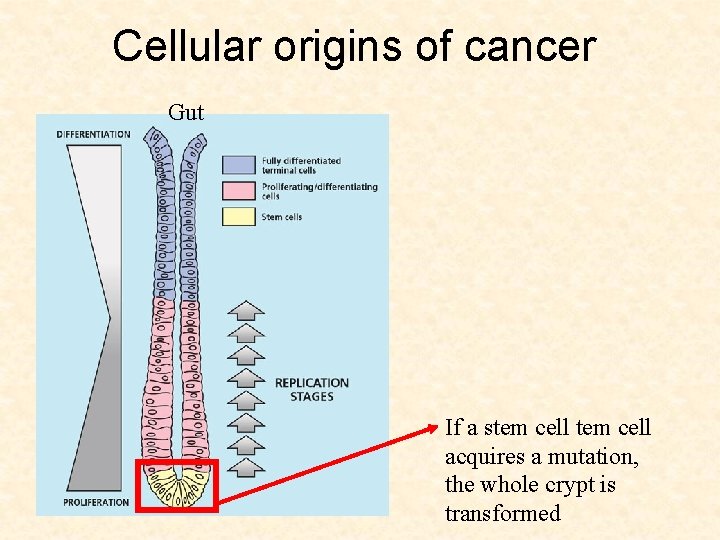
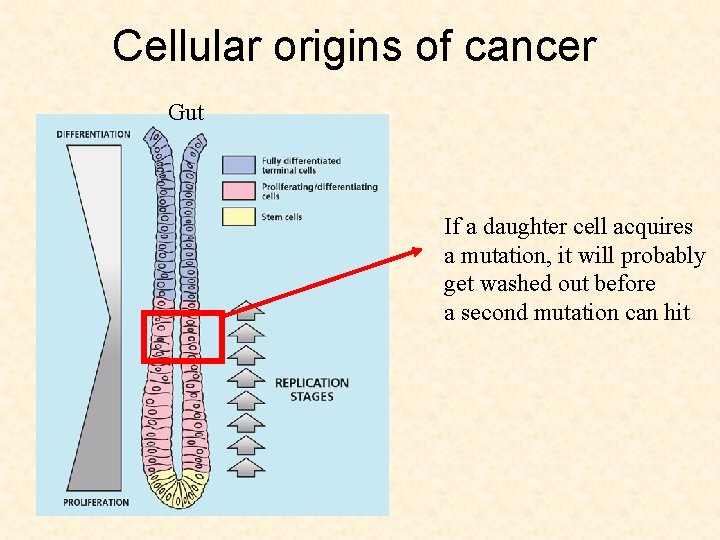
Cellular origins of cancer Gut If a stem cell acquires a mutation, the whole crypt is transformed

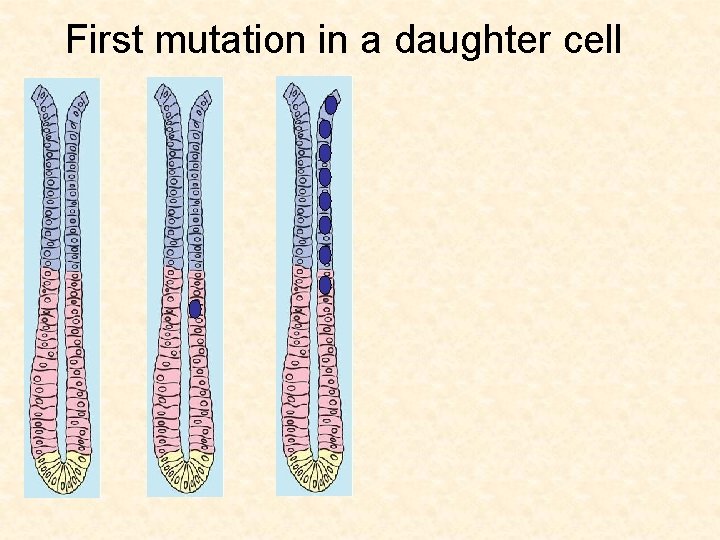
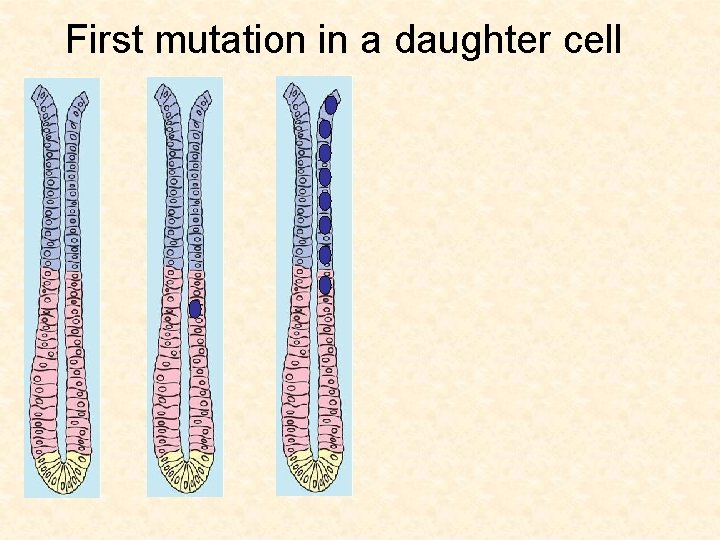
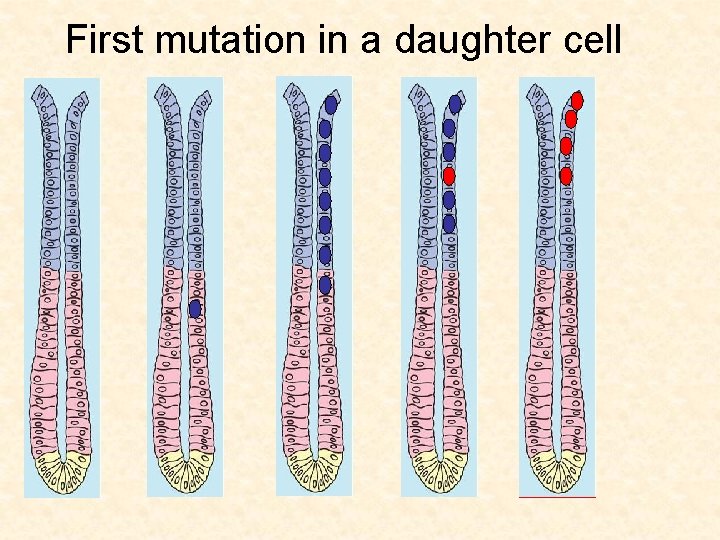
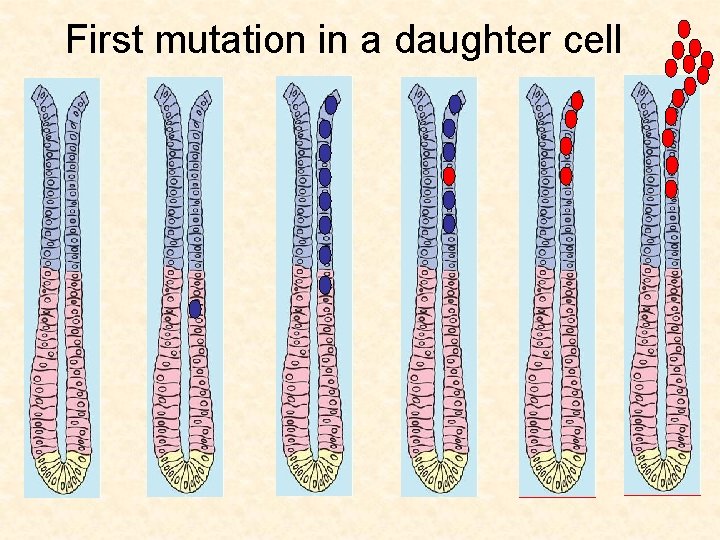
Cellular origins of cancer Gut If a daughter cell acquires a mutation, it will probably get washed out before a second mutation can hit

What is the cellular origin of cancer?

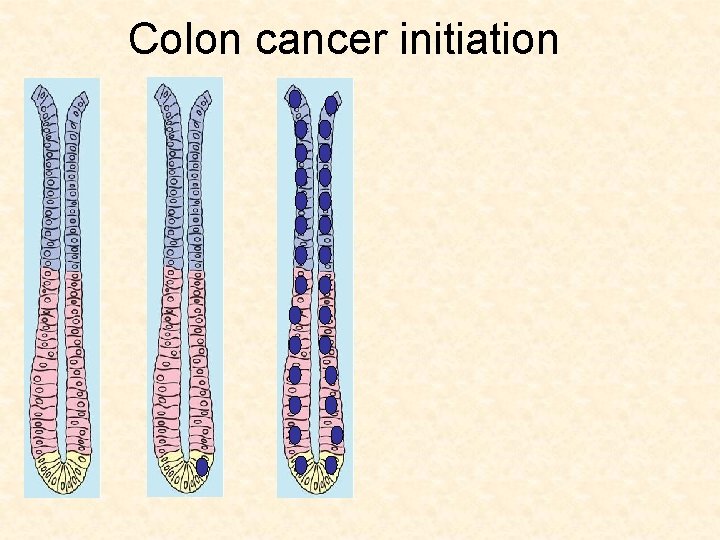
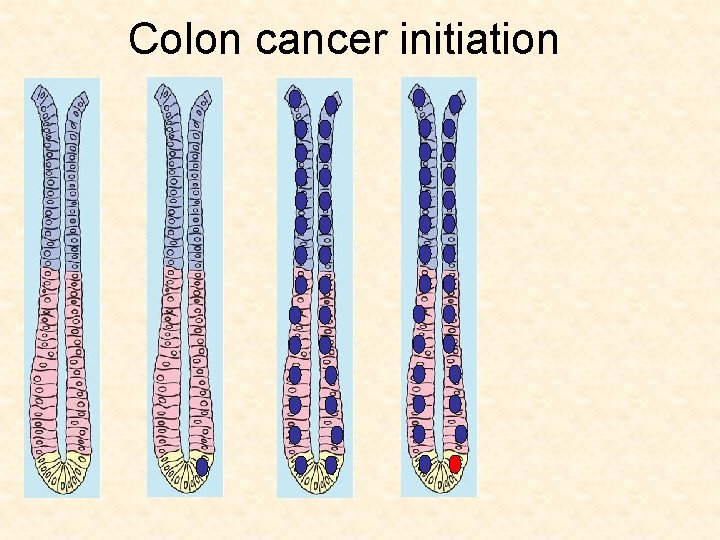
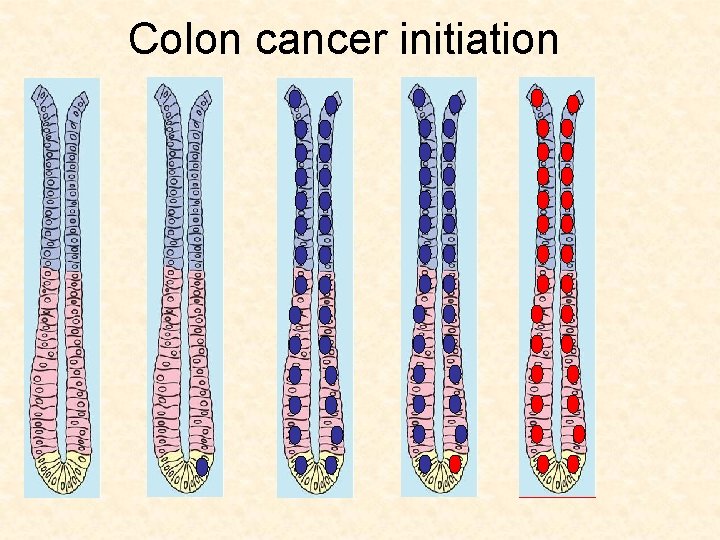
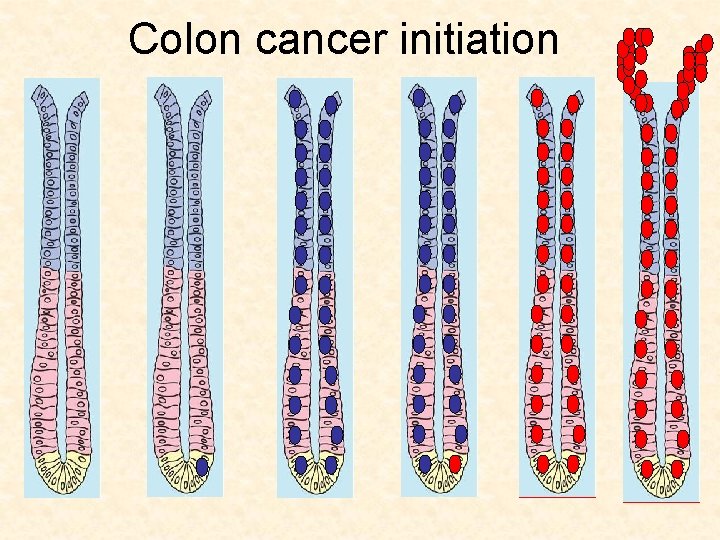
Colon cancer initiation

Colon cancer initiation

Colon cancer initiation

Colon cancer initiation

Colon cancer initiation

Colon cancer initiation

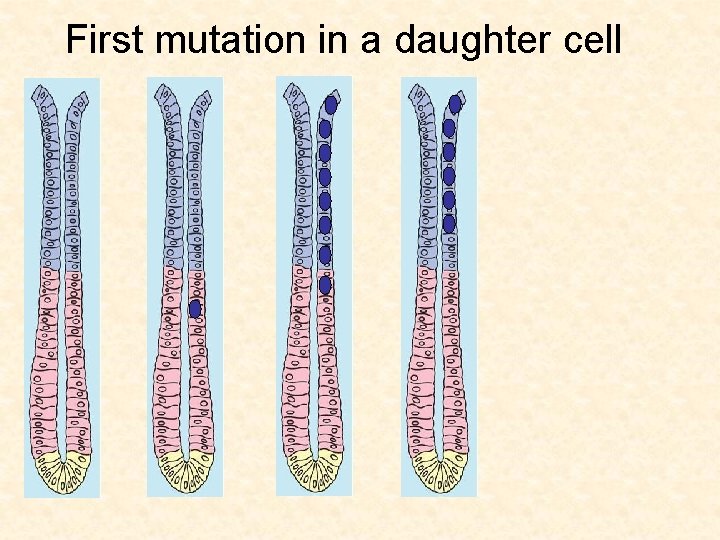
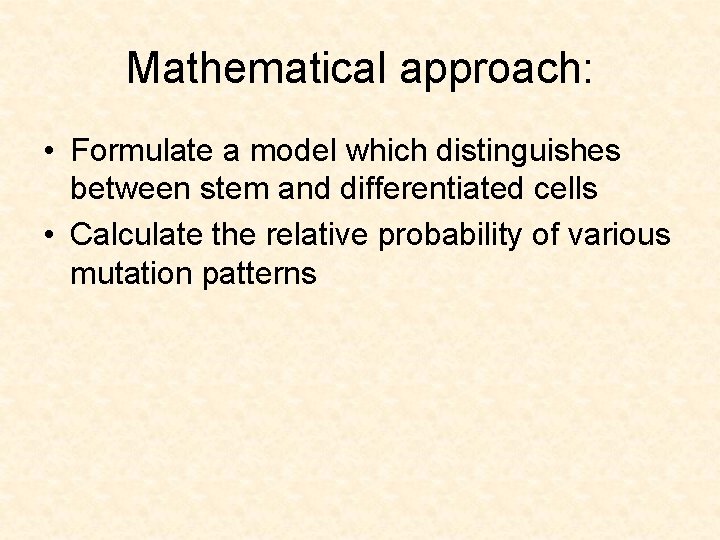
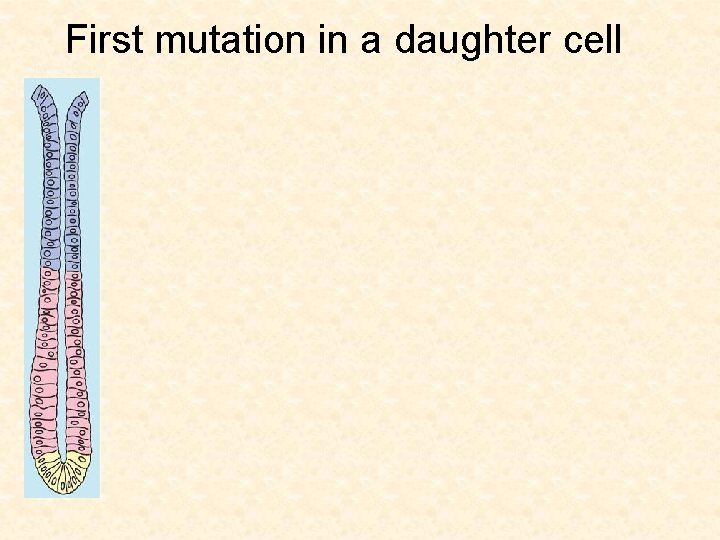
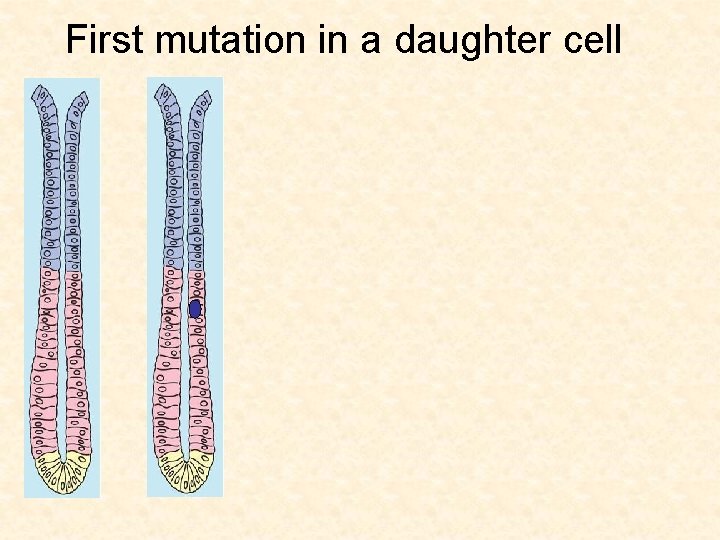
First mutation in a daughter cell

First mutation in a daughter cell

First mutation in a daughter cell

First mutation in a daughter cell

First mutation in a daughter cell

First mutation in a daughter cell

Cellular origins of cancer • The prevailing theory is that the mutations leading to cancer initiation occur is stem cells

Cellular origins of cancer • The prevailing theory is that the mutations leading to cancer initiation occur is stem cells • Therefore, all prevention and treatment strategies must target the stem cells

Cellular origins of cancer • The prevailing theory is that the mutations leading to cancer initiation occur is stem cells • Therefore, all prevention and treatment strategies must target the stem cells • Differentiated cells (most cells!) do not count

Mathematical approach: • Formulate a model which distinguishes between stem and differentiated cells • Calculate the relative probability of various mutation patterns

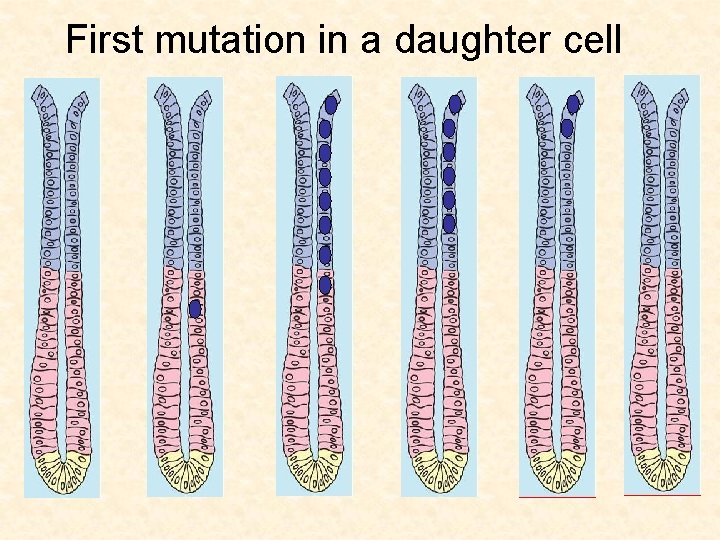
First mutation in a daughter cell

First mutation in a daughter cell

First mutation in a daughter cell

First mutation in a daughter cell

First mutation in a daughter cell

First mutation in a daughter cell

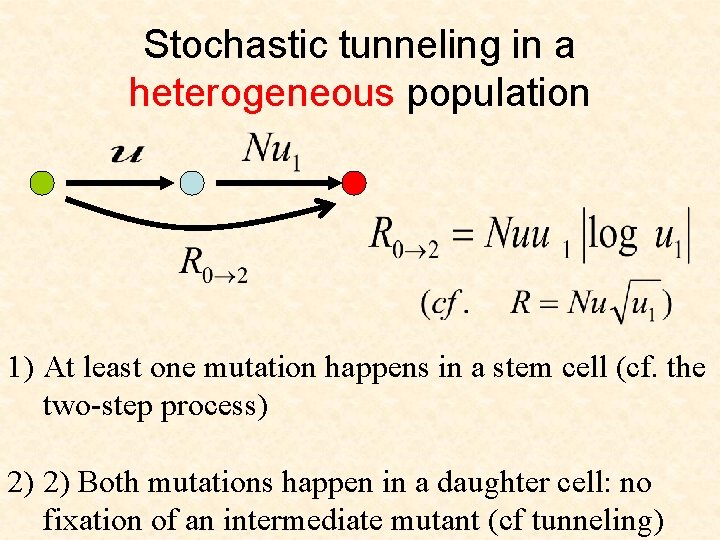
Stochastic tunneling in a heterogeneous population 1) At least one mutation happens in a stem cell (cf. the two-step process) 2) 2) Both mutations happen in a daughter cell: no fixation of an intermediate mutant (cf tunneling)

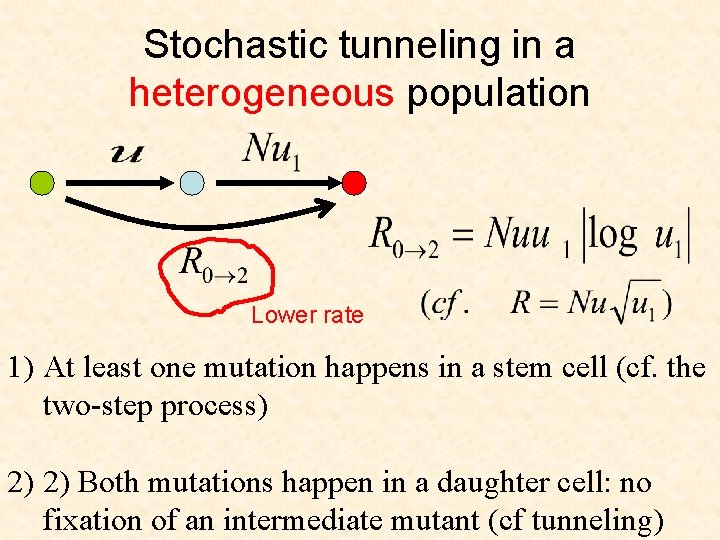
Stochastic tunneling in a heterogeneous population Lower rate 1) At least one mutation happens in a stem cell (cf. the two-step process) 2) 2) Both mutations happen in a daughter cell: no fixation of an intermediate mutant (cf tunneling)

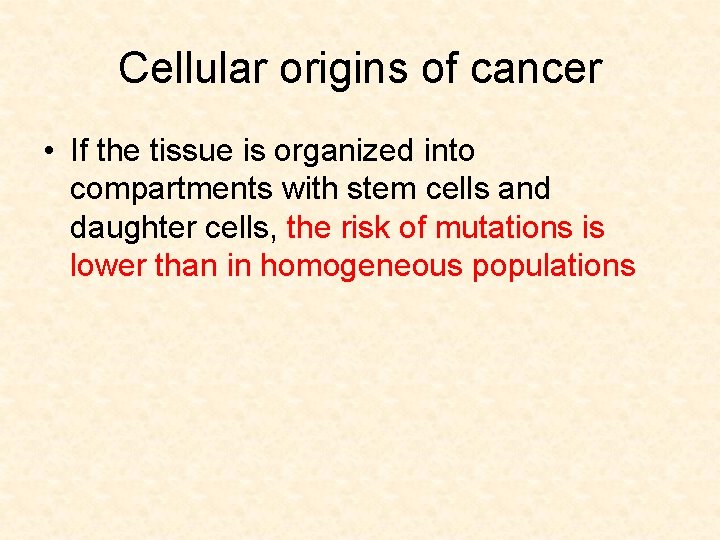
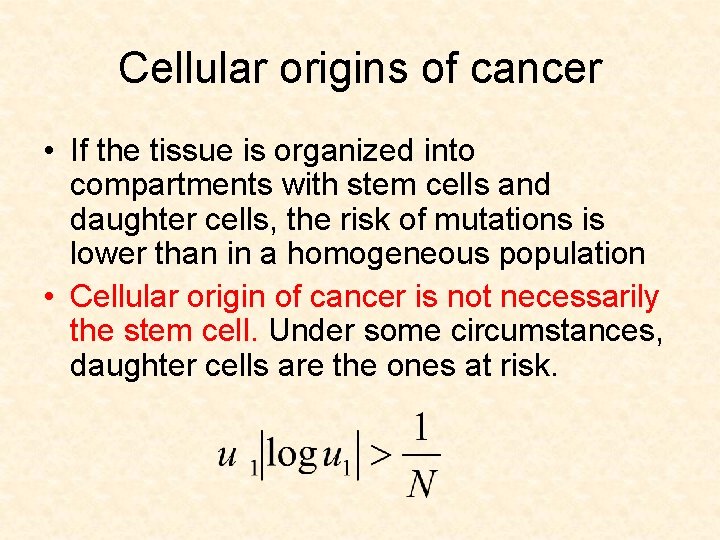
Cellular origins of cancer • If the tissue is organized into compartments with stem cells and daughter cells, the risk of mutations is lower than in homogeneous populations

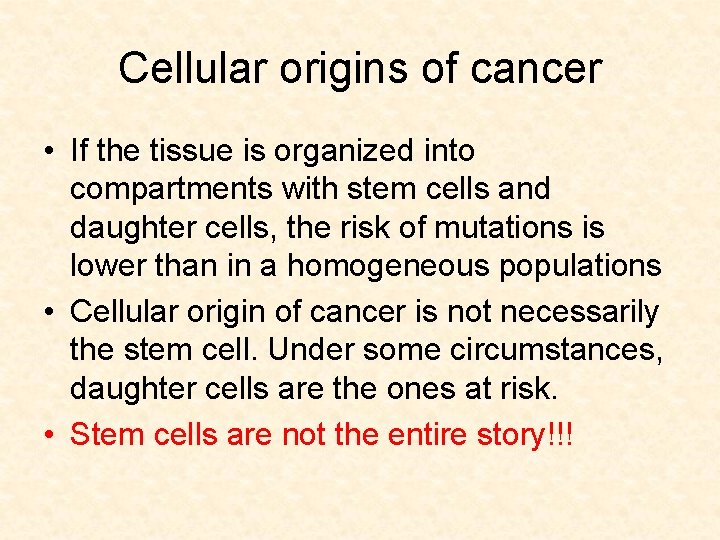
Cellular origins of cancer • If the tissue is organized into compartments with stem cells and daughter cells, the risk of mutations is lower than in a homogeneous population • Cellular origin of cancer is not necessarily the stem cell. Under some circumstances, daughter cells are the ones at risk.

Cellular origins of cancer • If the tissue is organized into compartments with stem cells and daughter cells, the risk of mutations is lower than in a homogeneous populations • Cellular origin of cancer is not necessarily the stem cell. Under some circumstances, daughter cells are the ones at risk. • Stem cells are not the entire story!!!

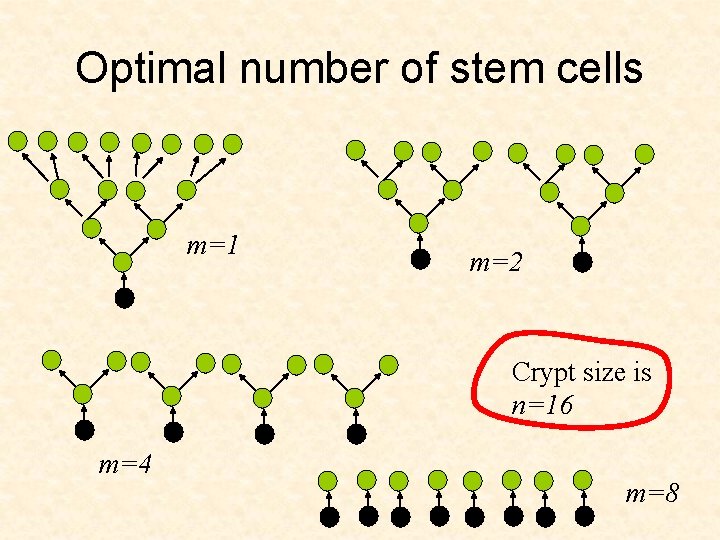
Optimal tissue architecture • How does tissue architecture help protect against cancer? • What are parameters of the architecture that minimize the risk of cancer? • How does protection against cancer change with the individual’s age?

Optimal number of stem cells m=1 m=2 Crypt size is n=16 m=4 m=8

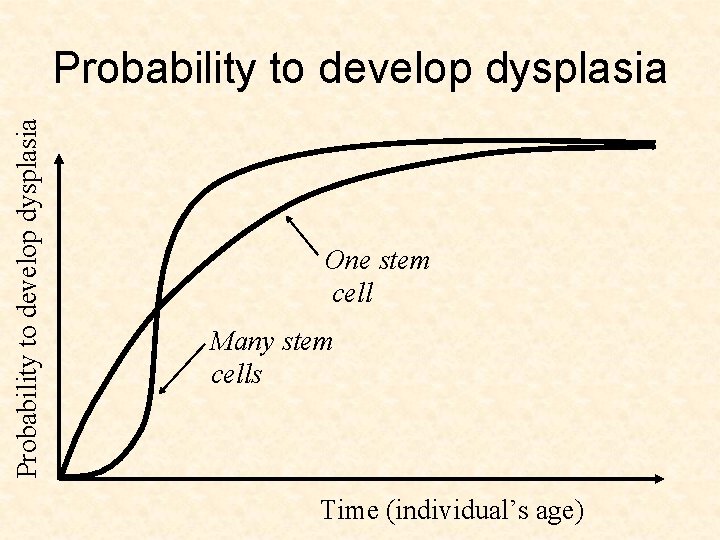
Probability to develop dysplasia One stem cell Many stem cells Time (individual’s age)

Probability to develop dysplasia The optimal solution is timedependent! Optimum: many stem cells One stem cell Many stem Optimum: cells one stem cell Time (individual’s age)

Optimization problem • The optimum number of stem cells is high in young age, and low in old age • Assume that tissue architecture cannot change with time: must choose a timeindependent solution • Selection mostly acts upon reproductive ages, so the preferred evolutionary strategy is to keep the risk of cancer low while the organism is young

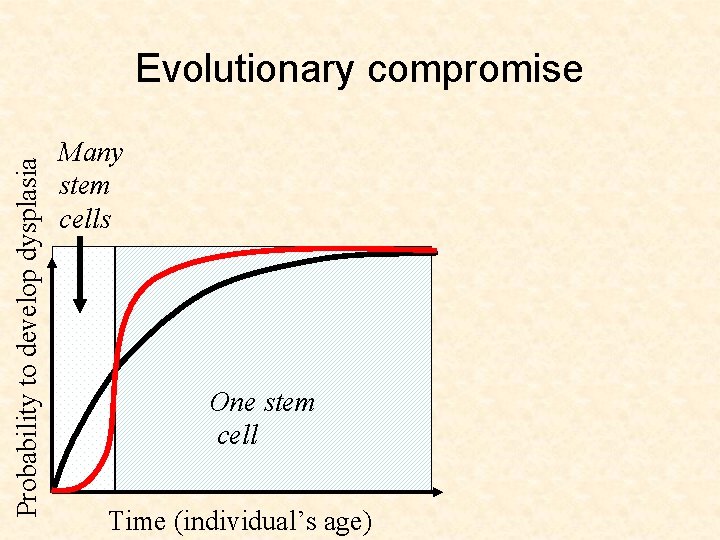
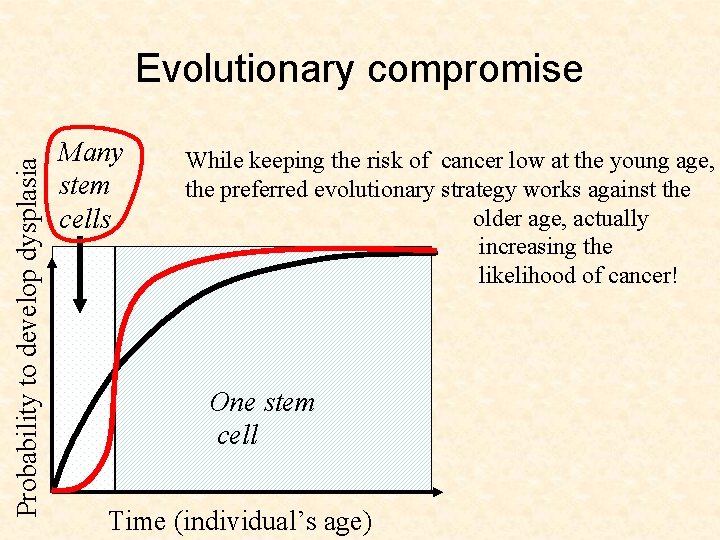
Probability to develop dysplasia Evolutionary compromise Many stem cells One stem cell Time (individual’s age)

Probability to develop dysplasia Evolutionary compromise Many stem cells While keeping the risk of cancer low at the young age, the preferred evolutionary strategy works against the older age, actually increasing the likelihood of cancer! One stem cell Time (individual’s age)

Cancer vs aging • Cancer and aging are two sides of the same coin…. .

Drug therapy and generation of resistance

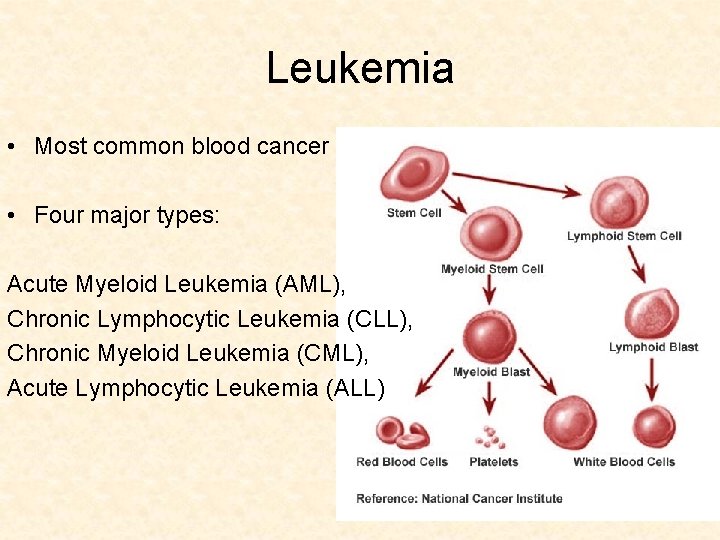
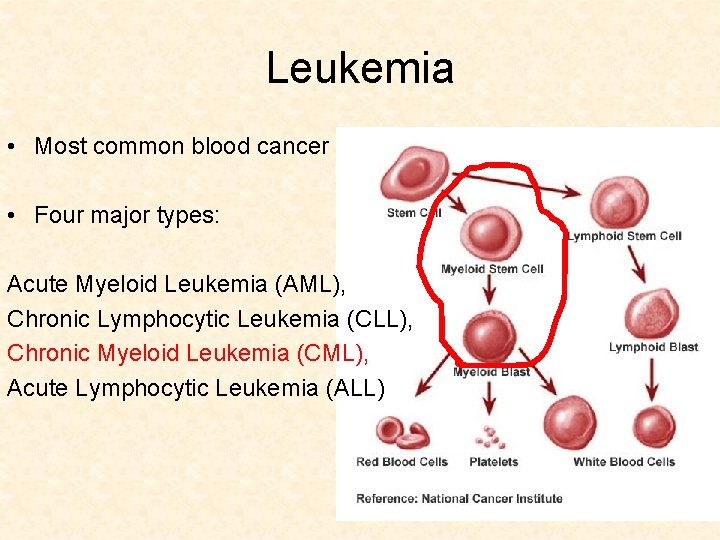
Leukemia • Most common blood cancer • Four major types: Acute Myeloid Leukemia (AML), Chronic Lymphocytic Leukemia (CLL), Chronic Myeloid Leukemia (CML), Acute Lymphocytic Leukemia (ALL)

Leukemia • Most common blood cancer • Four major types: Acute Myeloid Leukemia (AML), Chronic Lymphocytic Leukemia (CLL), Chronic Myeloid Leukemia (CML), Acute Lymphocytic Leukemia (ALL)

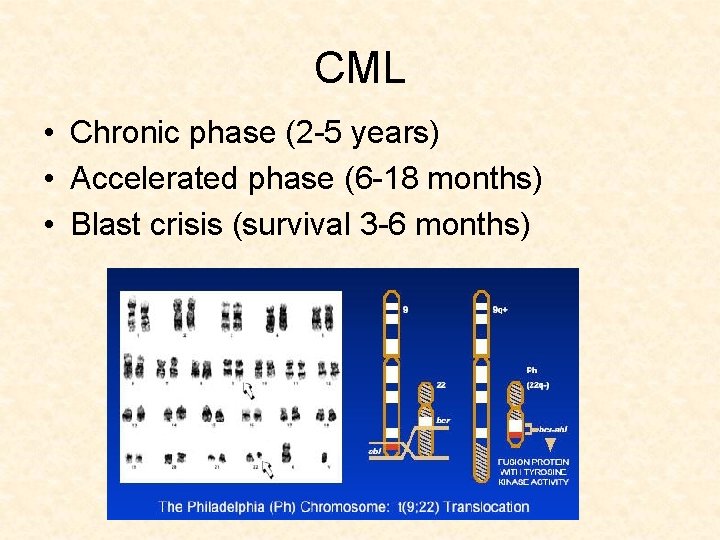
CML • Chronic phase (2 -5 years) • Accelerated phase (6 -18 months) • Blast crisis (survival 3 -6 months)

Targeted cancer drugs • Traditional drugs: very toxic agents that kill dividing cells

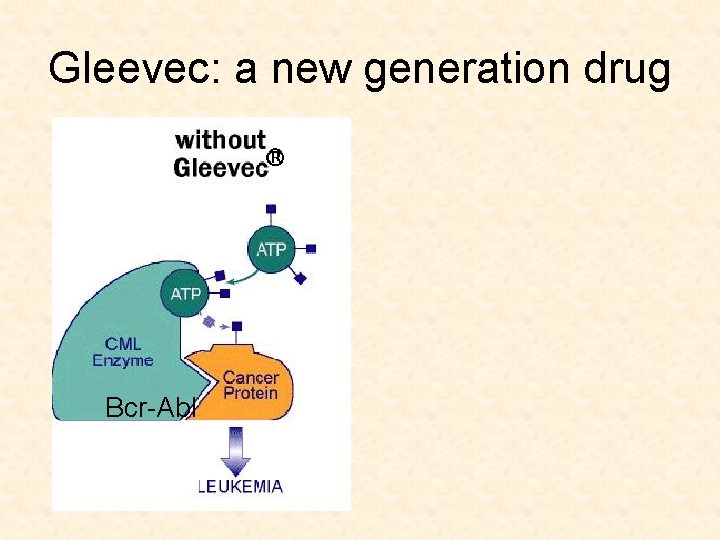
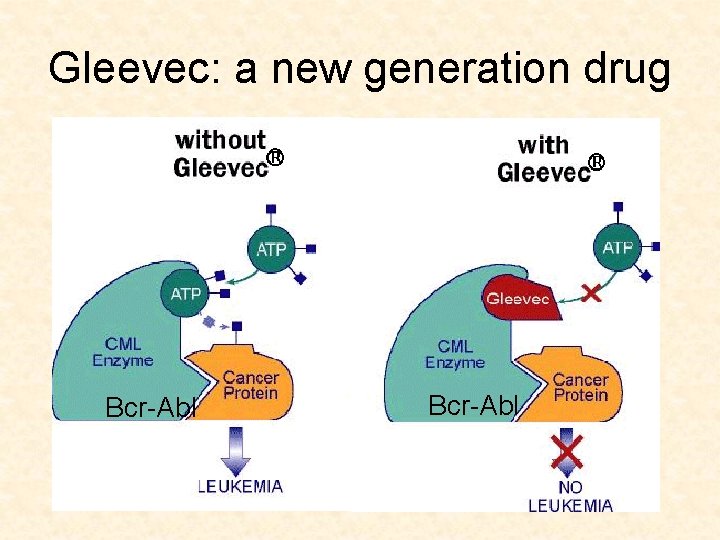
Targeted cancer drugs • Traditional drugs: very toxic agents that kill dividing cells • New drugs: small molecule inhibitors • Target the pathways which make cancerous cells cancerous (Gleevec)

Gleevec: a new generation drug Bcr-Abl

Gleevec: a new generation drug Bcr-Abl

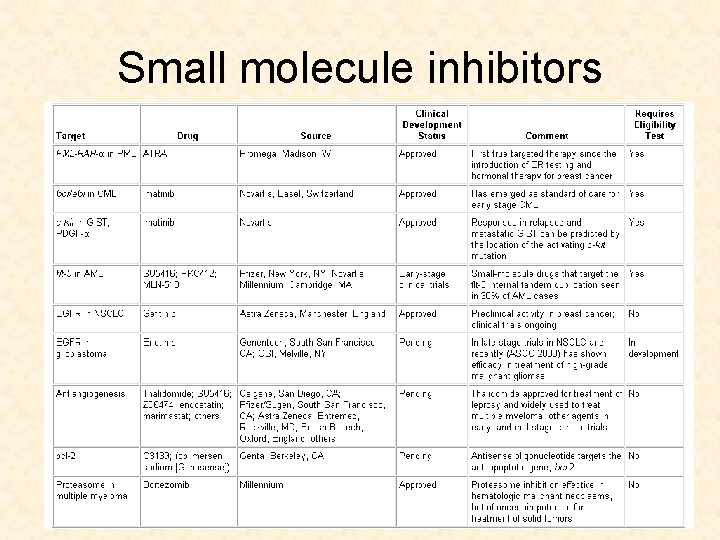
Small molecule inhibitors

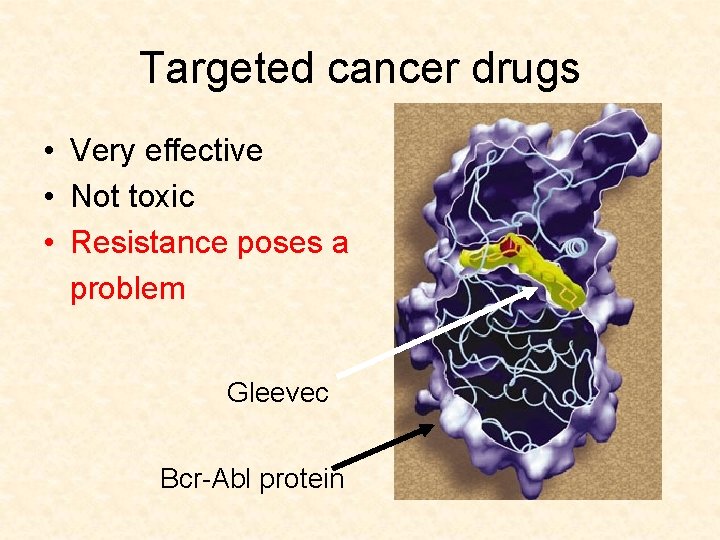
Targeted cancer drugs • Very effective • Not toxic

Targeted cancer drugs • Very effective • Not toxic • Resistance poses a problem Gleevec Bcr-Abl protein

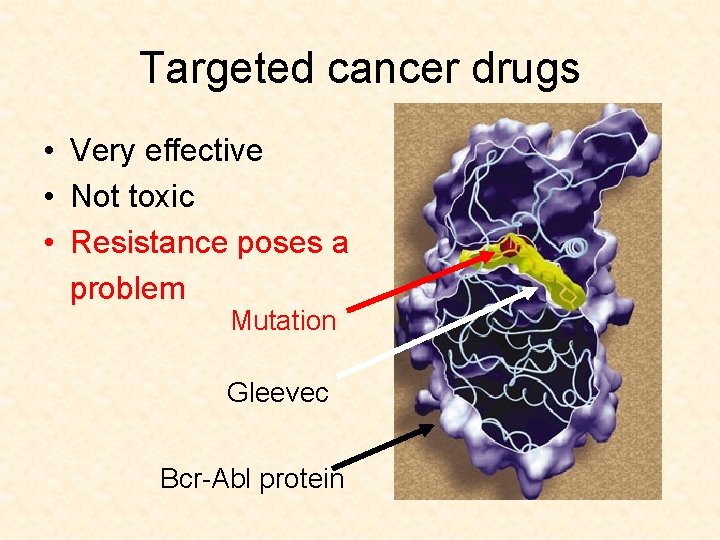
Targeted cancer drugs • Very effective • Not toxic • Resistance poses a problem Mutation Gleevec Bcr-Abl protein

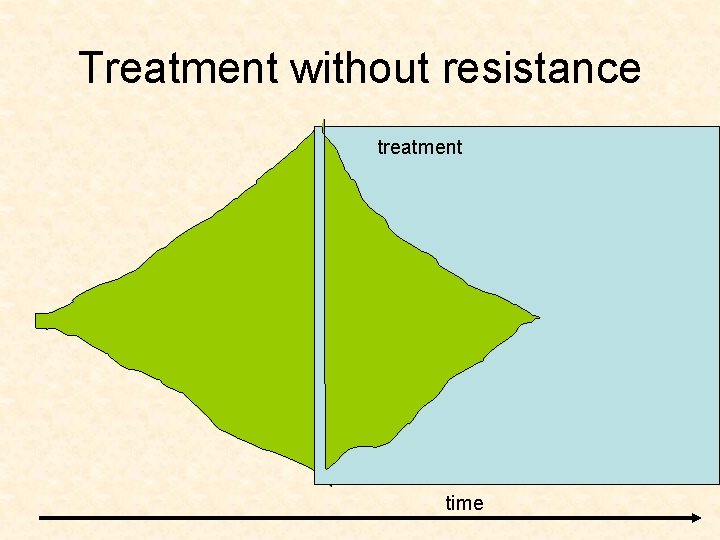
Treatment without resistance treatment time

Development of resistance treatment

How can one prevent resistance? • In HIV: treat with multiple drugs • It takes one mutation to develop resistance of one drug. It takes n mutations to develop resistance to n drugs. • Goal: describe the generation of resistance before and after therapy.

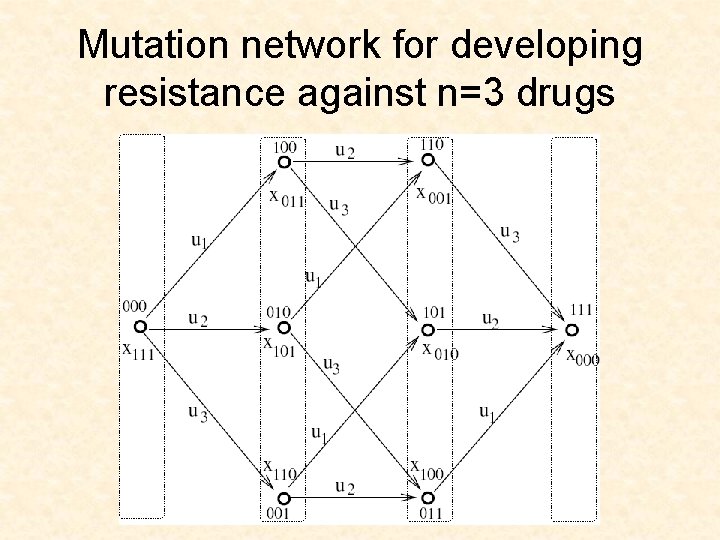
Mutation network for developing resistance against n=3 drugs

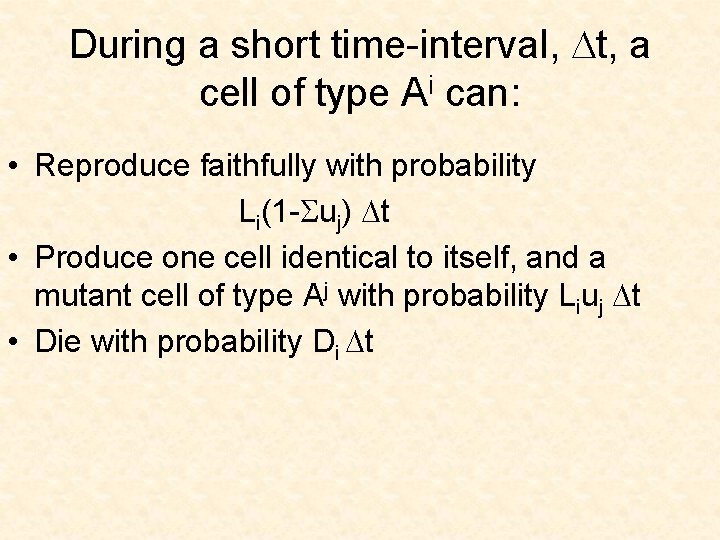
During a short time-interval, Dt, a cell of type Ai can: • Reproduce faithfully with probability Li(1 -Suj) Dt

During a short time-interval, Dt, a cell of type Ai can: • Reproduce faithfully with probability Li(1 -Suj) Dt • Produce one cell identical to itself, and a mutant cell of type Aj with probability Liuj Dt

During a short time-interval, Dt, a cell of type Ai can: • Reproduce faithfully with probability Li(1 -Suj) Dt • Produce one cell identical to itself, and a mutant cell of type Aj with probability Liuj Dt • Die with probability Di Dt

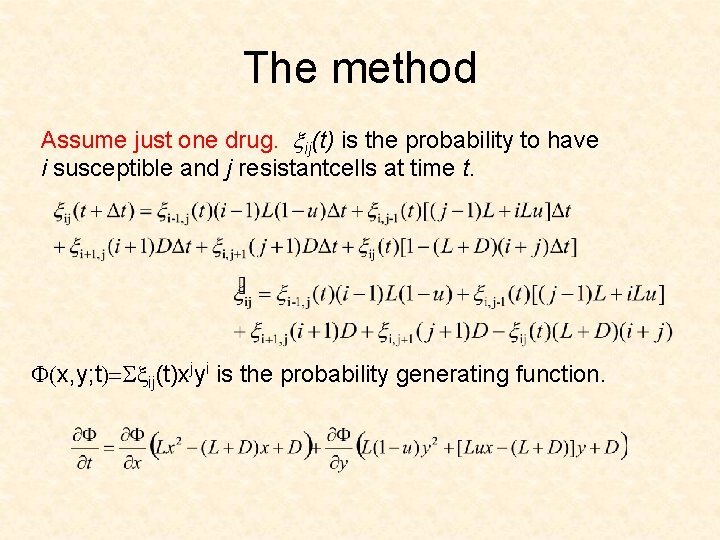
The method Assume just one drug. xij(t) is the probability to have i susceptible and j resistantcells at time t. F(x, y; t)=Sxij(t)xjyi is the probability generating function.

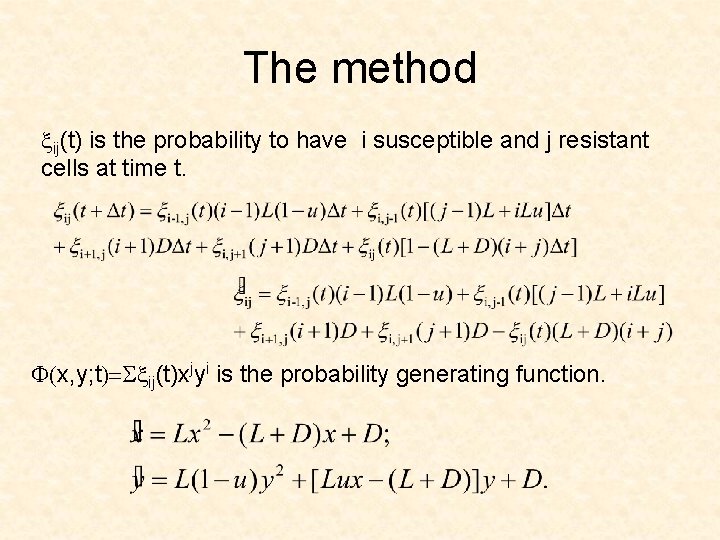
The method xij(t) is the probability to have i susceptible and j resistant cells at time t. F(x, y; t)=Sxij(t)xjyi is the probability generating function.

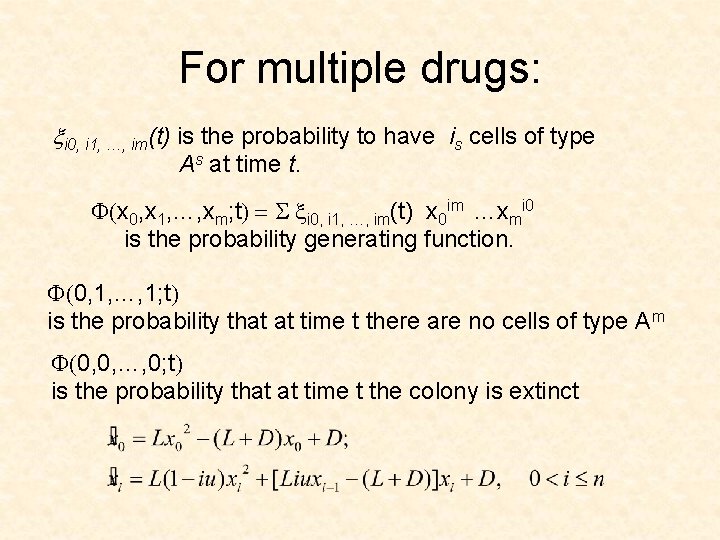
For multiple drugs: xi 0, i 1, …, im(t) is the probability to have is cells of type As at time t. F(x 0, x 1, …, xm; t) = S xi 0, i 1, …, im(t) x 0 im …xmi 0 is the probability generating function. F(0, 1, …, 1; t) is the probability that at time t there are no cells of type Am F(0, 0, …, 0; t) is the probability that at time t the colony is extinct

The method The probability that at time t the colony is extinct is F(0, 0, …, 0; t) =xn. M(t), where M is the initial # of cells and xn is the solution of The probability of treatment failure is

The questions: 1. Does resistance mostly arise before or after the start of treatment? 2. How does generation of resistance depend on the properties of cancer growth (high turnover D~L vs low turnover D<<L) 3. How does the number of drugs influence the success of treatment?

1. How important is pre-existence of mutants?

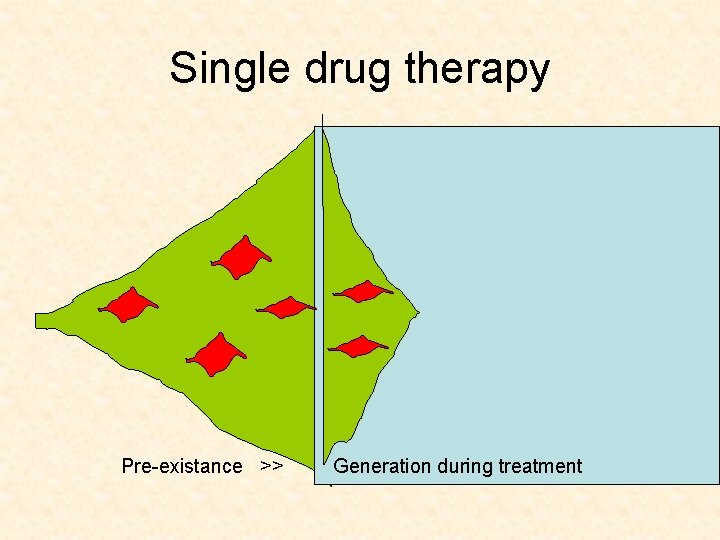
Single drug therapy

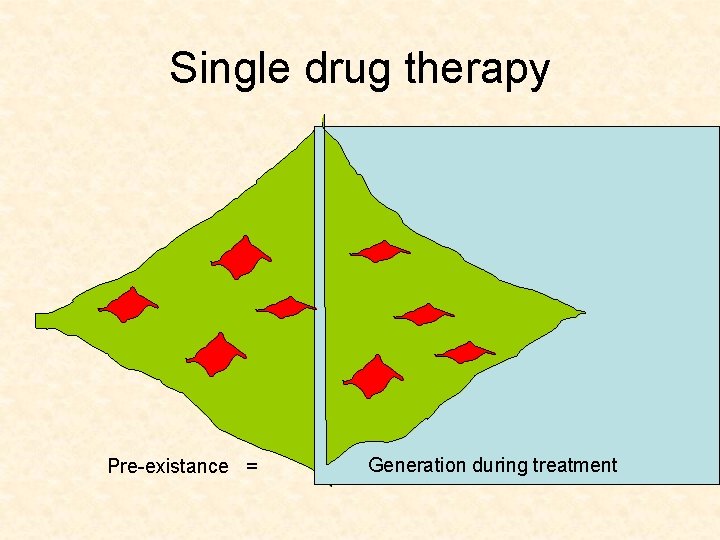
Single drug therapy Pre-existance = Generation during treatment

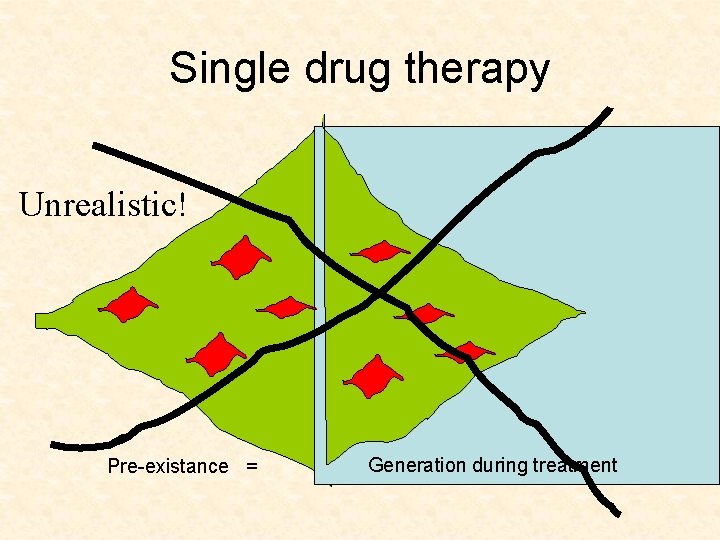
Single drug therapy Unrealistic! Pre-existance = Generation during treatment

Single drug therapy Pre-existance >> Generation during treatment

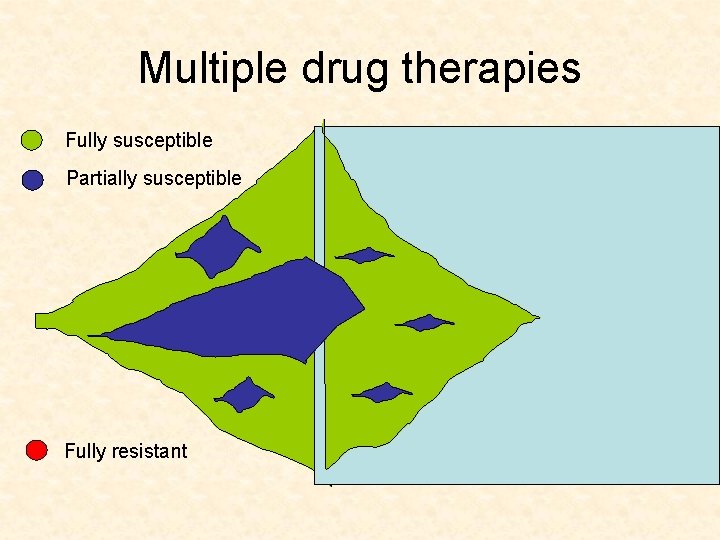
Multiple drug therapies Fully susceptible Partially susceptible Fully resistant

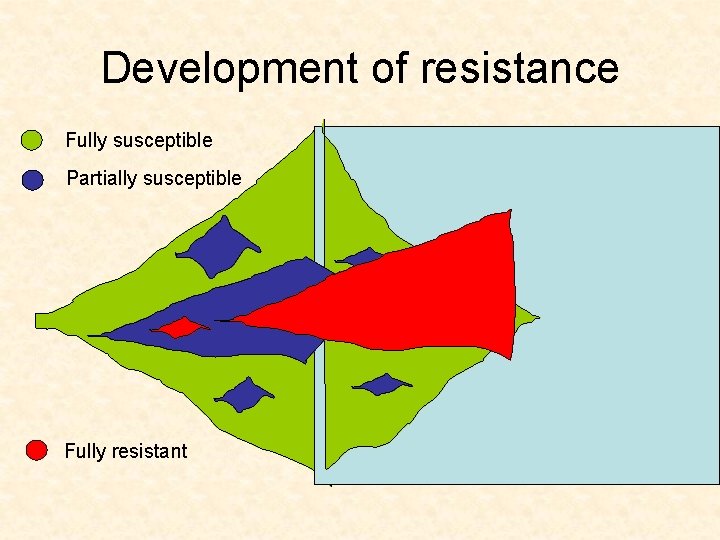
Development of resistance Fully susceptible Partially susceptible Fully resistant

1. How important is pre-existence of resistant mutants? For both single- and multiple-drug therapies, resistant mutants are likely to be produced before start of treatment, and not in the course of treatment

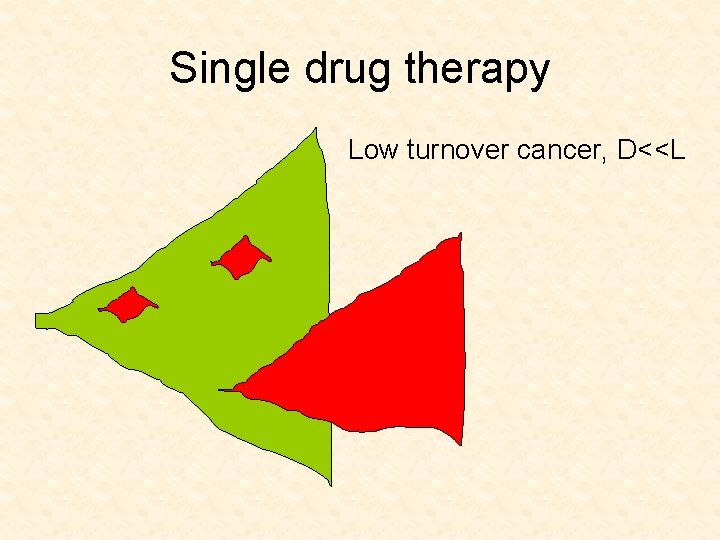
2. How does generation of resistance depend on the turnover rate of cancer? • Low turnover (growth rate>>death rate) Fewer cell divisions needed to reach a certain size • High turnover (growth rate~death rate) Many cell divisions needed to reach a certain size

Single drug therapy Low turnover cancer, D<<L

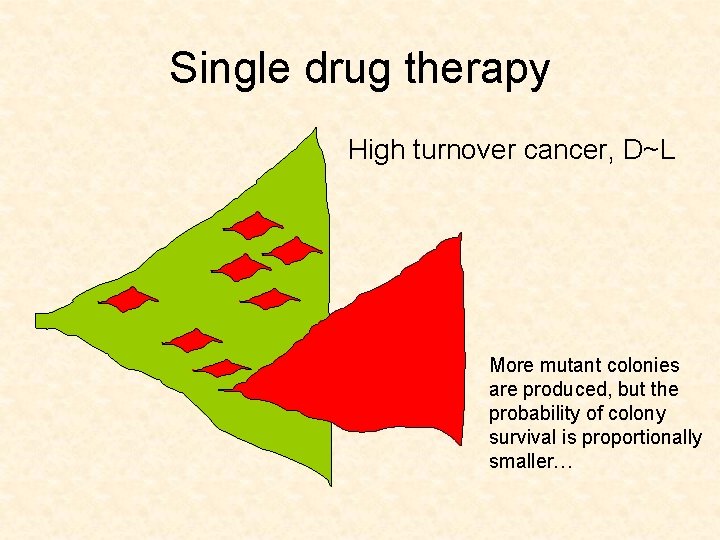
Single drug therapy High turnover cancer, D~L More mutant colonies are produced, but the probability of colony survival is proportionally smaller…

2. How does generation of resistance depend on the turnover rate of cancer? • Single drug therapies: the production of mutants is independent of the turnover

2. How does generation of resistance depend on the turnover rate of cancer? • Single drug therapies: the production of mutants is independent of the turnover • Multiple drug therapies: the production of mutants is much larger for cancers with a high turnover

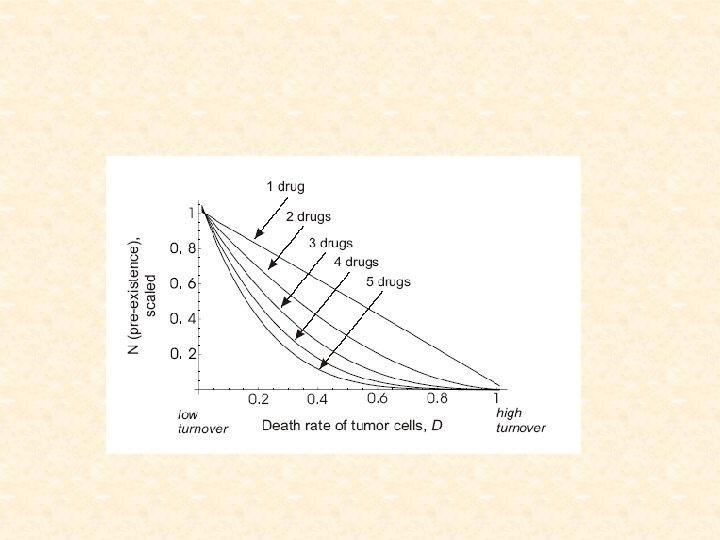
3. The size of failure • Suppose we start treatment at size N • Calculate the probability of treatment failure • Find the size at which the probability of failure is d=0. 01

3. The size of failure • Suppose we start treatment at size N • Calculate the probability of treatment failure • Find the size at which the probability of failure is d=0. 01 • The size of failure increases with # of drugs and decreases with mutation rate

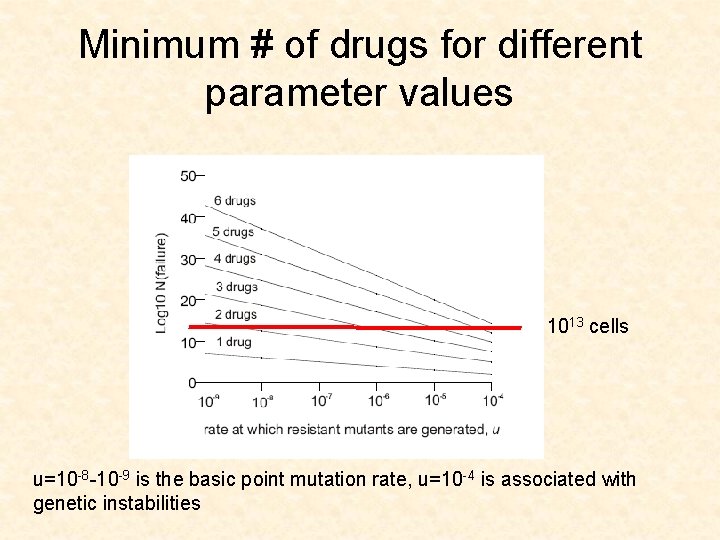
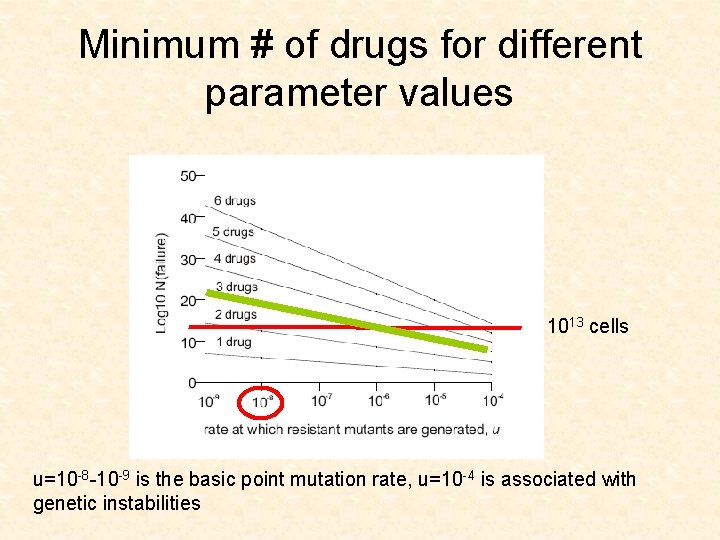
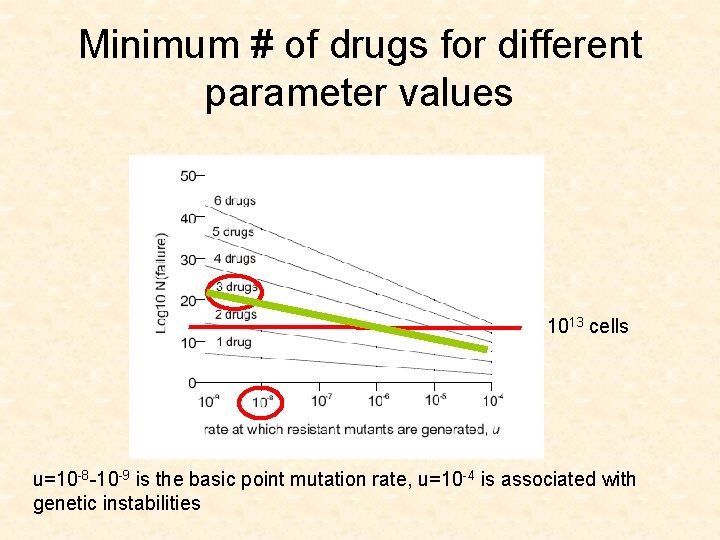
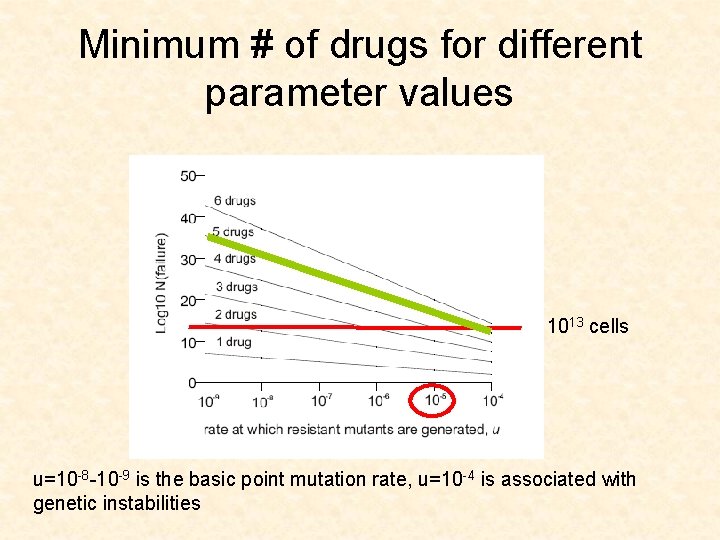
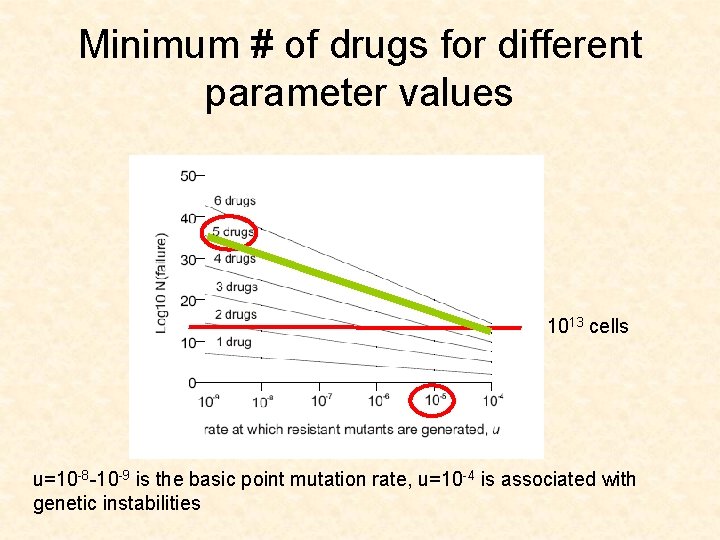
Minimum # of drugs for different parameter values 1013 cells u=10 -8 -10 -9 is the basic point mutation rate, u=10 -4 is associated with genetic instabilities

Minimum # of drugs for different parameter values 1013 cells u=10 -8 -10 -9 is the basic point mutation rate, u=10 -4 is associated with genetic instabilities

Minimum # of drugs for different parameter values 1013 cells u=10 -8 -10 -9 is the basic point mutation rate, u=10 -4 is associated with genetic instabilities

Minimum # of drugs for different parameter values 1013 cells u=10 -8 -10 -9 is the basic point mutation rate, u=10 -4 is associated with genetic instabilities

Minimum # of drugs for different parameter values 1013 cells u=10 -8 -10 -9 is the basic point mutation rate, u=10 -4 is associated with genetic instabilities

CML leukemia • • Gleevec u=10 -8 -10 -9 D/L between 0. 1 and 0. 5 (low turnover) Size of advanced cancers is 1013 cells

Log size of treatment failure u=10 -8 u=10 -6

Application for CML • The model suggests that 3 drugs are needed to push the size of failure (1% failure) up to 1013 cells

Conclusions • Main concept: cancer is a highly structured evolutionary process • Main tool: stochastic processes on selection-mutation networks • We addressed questions of cellular origins of cancer and generation of drug resistance • There are many more questions in cancer research…




Multiple drug treatments • For fast turnover cancers, adding more drugs will not prevent generation of resistance

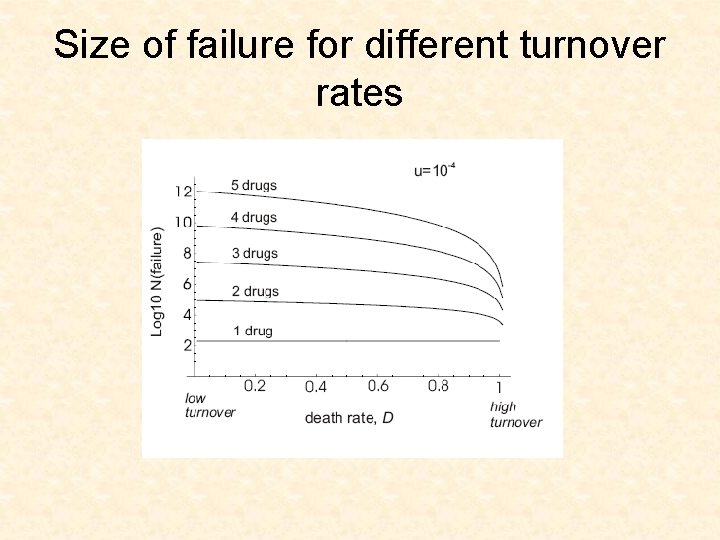
Size of failure for different turnover rates
 Howard university cancer center
Howard university cancer center Thermoreceptors
Thermoreceptors Chapter 29 somatic symptom and dissociative disorders
Chapter 29 somatic symptom and dissociative disorders Diploid cell
Diploid cell Dissociative
Dissociative Distinguish between general senses and special senses.
Distinguish between general senses and special senses. Nervous system main division
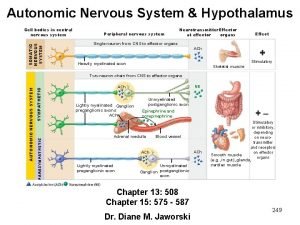
Nervous system main division Autonomic nervous system pathway
Autonomic nervous system pathway Forensic
Forensic Somatic senses
Somatic senses Spinal cord anatomy
Spinal cord anatomy Prevertebral ganglia
Prevertebral ganglia Hoarding disorder بالعربي
Hoarding disorder بالعربي Sns pg
Sns pg Alpha and gamma motor neurons
Alpha and gamma motor neurons Classification of somatic senses
Classification of somatic senses Haploid and diploid venn diagram
Haploid and diploid venn diagram Loose association speech
Loose association speech Rib raising omt
Rib raising omt Site of somatic motor neuron cell bodies
Site of somatic motor neuron cell bodies Orthomode transducer tutorial
Orthomode transducer tutorial Somatic
Somatic Erbs palsy
Erbs palsy Process of somatic embryogenesis
Process of somatic embryogenesis Site:slidetodoc.com
Site:slidetodoc.com Rib somatic dysfunction
Rib somatic dysfunction Why dna is more stable than rna
Why dna is more stable than rna Somatic motor neuron
Somatic motor neuron Ornithine cycle
Ornithine cycle Affinity maturation somatic hypermutation
Affinity maturation somatic hypermutation Lmnl vs umnl
Lmnl vs umnl Somatic motor division
Somatic motor division Somatic vs factitious
Somatic vs factitious Differentiate rigor mortis from cadaveric spasm
Differentiate rigor mortis from cadaveric spasm Autonomic ganglia
Autonomic ganglia Sensory modality examples
Sensory modality examples Gamete vs somatic cell
Gamete vs somatic cell Germ cell vs somatic cells
Germ cell vs somatic cells Neurotransmitter in somatic nervous system
Neurotransmitter in somatic nervous system Ans
Ans Isodiametric hair
Isodiametric hair What is the medulla of the hair
What is the medulla of the hair Somatic motor function
Somatic motor function Somatic hypermutation
Somatic hypermutation Cortical fusi definition forensics
Cortical fusi definition forensics Somatic nervous system
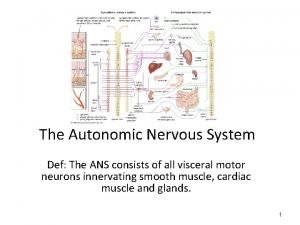
Somatic nervous system Autonomic nervous system def
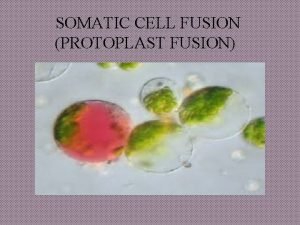
Autonomic nervous system def Protoplast fusion
Protoplast fusion Neuro tract
Neuro tract Chemofusion
Chemofusion Urt
Urt Somatic symptoms
Somatic symptoms Somatic vs autonomic nervous system
Somatic vs autonomic nervous system Somatic tremor artifact
Somatic tremor artifact Somatic pathway
Somatic pathway Somatic ganglion
Somatic ganglion Mitosis somatic
Mitosis somatic Somaclonal variation
Somaclonal variation Somatic recombination
Somatic recombination Dura mater meaning
Dura mater meaning Nursing diagnosis of somatoform disorder
Nursing diagnosis of somatoform disorder Vindicate mnemonic
Vindicate mnemonic Somatic symptom
Somatic symptom Somatic
Somatic Somatic hybridization diagram
Somatic hybridization diagram Tabes dorsalis
Tabes dorsalis Somatic nervous system
Somatic nervous system Somatic sx disorder
Somatic sx disorder Pns
Pns Somatic
Somatic Pain ascending pathway
Pain ascending pathway Somatic cell
Somatic cell Somatic mutation
Somatic mutation An ipsilateral intersegmental spinal somatic reflex
An ipsilateral intersegmental spinal somatic reflex Primitive node
Primitive node Somatic cells are also called
Somatic cells are also called Chapter 17 somatic symptom disorders
Chapter 17 somatic symptom disorders Cell division vocabulary
Cell division vocabulary Chapter 24 the immune and lymphatic systems and cancer
Chapter 24 the immune and lymphatic systems and cancer What empties into the left subclavian vein
What empties into the left subclavian vein Sierzputowska natalia
Sierzputowska natalia Natalia olmo
Natalia olmo św. natalia 27 lipca
św. natalia 27 lipca Sverker johansson wikipedia
Sverker johansson wikipedia Natalia dorota partyka
Natalia dorota partyka Community health workers
Community health workers Dr natalia widiasih
Dr natalia widiasih Discord
Discord Natalia s'est le bras.
Natalia s'est le bras. Natalia yazigi md
Natalia yazigi md Natalia sierzputowska gonciarz
Natalia sierzputowska gonciarz Natalia mara
Natalia mara Natalia humphreys
Natalia humphreys Zdarzenie prawne
Zdarzenie prawne Natalia vesselova
Natalia vesselova Natalia mara
Natalia mara Natalia laverde
Natalia laverde Natalia bacikova
Natalia bacikova Natalia vassilieva
Natalia vassilieva Natalia mara
Natalia mara Natalia rak wybór
Natalia rak wybór Natalia mara
Natalia mara Rociguat
Rociguat Dzial sztuk plastycznych obejmujacy techniki odbitkowe
Dzial sztuk plastycznych obejmujacy techniki odbitkowe Natalia tokarova
Natalia tokarova Moskwa stanisław młodożeniec
Moskwa stanisław młodożeniec Rimas con natalia
Rimas con natalia Szyszak hadesa
Szyszak hadesa Meena makary
Meena makary Filme pornô as brasileirinhas
Filme pornô as brasileirinhas Natalia knoblock
Natalia knoblock Natalia komendera
Natalia komendera Natalia vassilieva
Natalia vassilieva Natália bánesová
Natália bánesová Natalia szpeflik
Natalia szpeflik Natalia gralewska
Natalia gralewska Edyta żukowska logopeda
Edyta żukowska logopeda Natalia tamblay
Natalia tamblay Natalia milas
Natalia milas Natalia bryant
Natalia bryant Natalia narloch
Natalia narloch Nicole rafidi
Nicole rafidi David galindo tceq
David galindo tceq Natalia konradus
Natalia konradus Kekebalan seluler melibatkan sel t yang bertugas .
Kekebalan seluler melibatkan sel t yang bertugas . Natalia konradus
Natalia konradus Atribut psikologi klinis
Atribut psikologi klinis Natalia goncharova cyclist
Natalia goncharova cyclist Natalia caceres salmos
Natalia caceres salmos Dominika mazurek
Dominika mazurek Contoh blue print psikologi
Contoh blue print psikologi świecznik rozgałęziony
świecznik rozgałęziony Natalia nox
Natalia nox The cyclist natalia goncharova
The cyclist natalia goncharova Natalia mariño castro
Natalia mariño castro Natalia ratajczyk
Natalia ratajczyk Natalia becerra
Natalia becerra Natalia sierzputowska wiek
Natalia sierzputowska wiek Carmen osovnikar
Carmen osovnikar Pengukuran eksternal
Pengukuran eksternal Natalia grzomba
Natalia grzomba Natalia aguayo
Natalia aguayo Natalia gralewska
Natalia gralewska Natalia gralewska
Natalia gralewska Escuela de la lonja
Escuela de la lonja Natalia emblem
Natalia emblem Natalia polyakova
Natalia polyakova