Solving Word Equation Word equations scare me Word














- Slides: 20

Solving Word Equation

“Word equations” scare me!”
“Word Equations” make me nervous!”

“Word Equations? I just skip them!”

Don’t Worry! With just 4 easy steps, you can become… a Word Equation. Whiz!

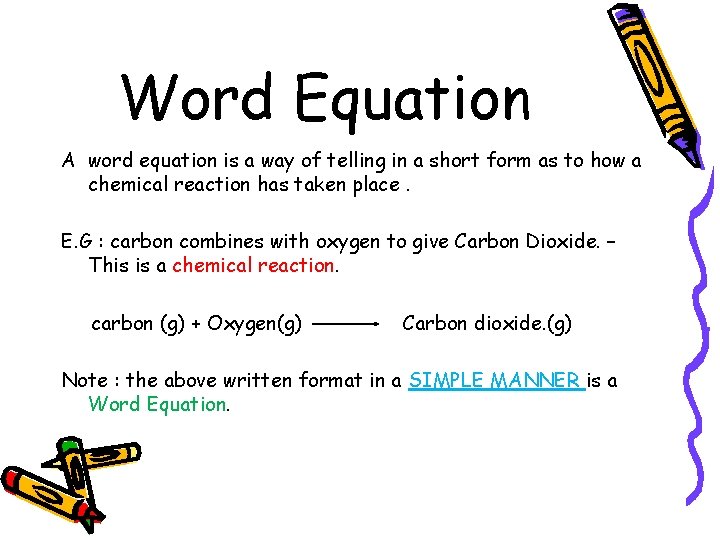
Word Equation A word equation is a way of telling in a short form as to how a chemical reaction has taken place. E. G : carbon combines with oxygen to give Carbon Dioxide. – This is a chemical reaction. carbon (g) + Oxygen(g) Carbon dioxide. (g) Note : the above written format in a SIMPLE MANNER is a Word Equation.

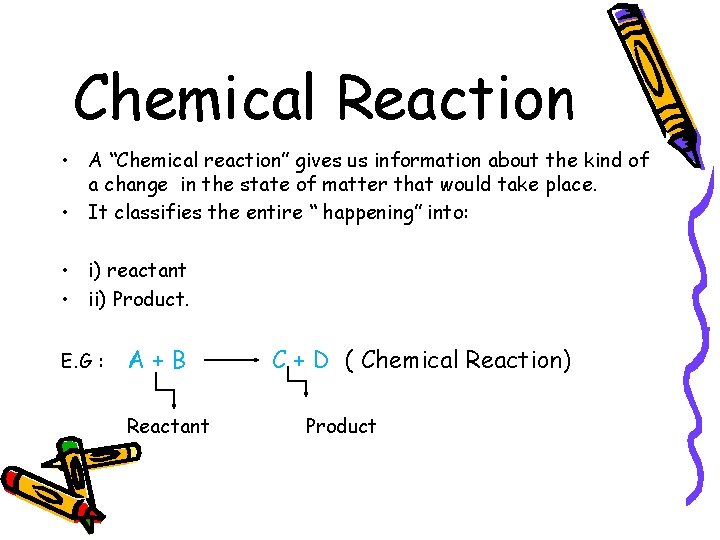
Chemical Reaction • A “Chemical reaction” gives us information about the kind of a change in the state of matter that would take place. • It classifies the entire “ happening” into: • i) reactant • ii) Product. E. G : A+B Reactant C + D ( Chemical Reaction) Product

Look for clues. Reactants : This will tell us what are the “things” that will combine. E. g. A + B Products: This is the “end point” telling us what new substances are formed. E. g. C + D

Which is R & P? ? • Carbon + Oxygen Carbon dioxide • Magnesium + Hydro chloric Acid Magnesium Chloride and Hydrogen. ( can you recall where this was used? ? )

Word Equation contd. . • Some times to write a chemical equation easily, we use simple ways. Such simple ways of writing Equations is done by using “ Chemical Symbols”. • These Symbols are short forms used. C + 02 CO 2 Short form for _______? Short for ______?

Task 1 1. Hydrogen and oxygen combine to give water. 2. H 2 + O 2 H 2 O 3. Iron in presence of Moist air ( oxygen ) gives Rust “ For the above mentioned , write as word equation. Classify each of them into a reactant and product”. Self check the answers.

Something “New” • Hydrochloric acid • Sulphuric Acid • Nitric Acid HCl H 2 SO 4 HNO 3 These are the names of most commonly used acids in the laboratory along with their SYMBOLS.

Reactions can be further divided into 2 types : 1. Reversible Reactions 2. Irreversible Reactions.

Work it out!

Task 2 Research Activity: “ Find out from the Net : • Meaning of reversible& irreversible reactions. • Give 5 examples for each also represent them in the form of word equation.

Give it a try! http: //www. factmonster. com/chemistry/ simlab/carbonatept 1. html

Recall the Journey… • What did you understand from the experiment you have done? • Did it give you a feel as if you were doing something? • 3 things you have learned from this activity.
We did it! We are Word equation Whizzes!

Here are the steps once more: Step 1 – UNDERSTAND the reaction. Step 2 – Write in WE (find which is R and P). Step 3 – Use Symbols! Step 4 – LOOK BACK.

Any Questions? ?