SOLVING PROBLEMS NOT AT EQUILIBRIUM The Reaction Quotient

SOLVING PROBLEMS NOT AT EQUILIBRIUM

The Reaction Quotient Ratio of Products to Reactants of a Reaction NOT NECESSARILY at equilibrium. The formula is the same as the equilibrium constant. Q = [Products]coefficient [Reactants] coefficient Compare value to equilibrium constant
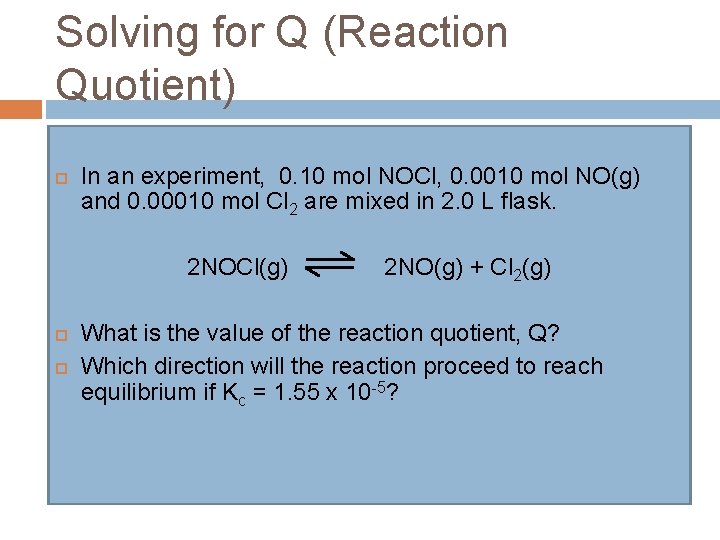
Solving for Q (Reaction Quotient) In an experiment, 0. 10 mol NOCl, 0. 0010 mol NO(g) and 0. 00010 mol Cl 2 are mixed in 2. 0 L flask. 2 NOCl(g) 2 NO(g) + Cl 2(g) What is the value of the reaction quotient, Q? Which direction will the reaction proceed to reach equilibrium if Kc = 1. 55 x 10 -5?
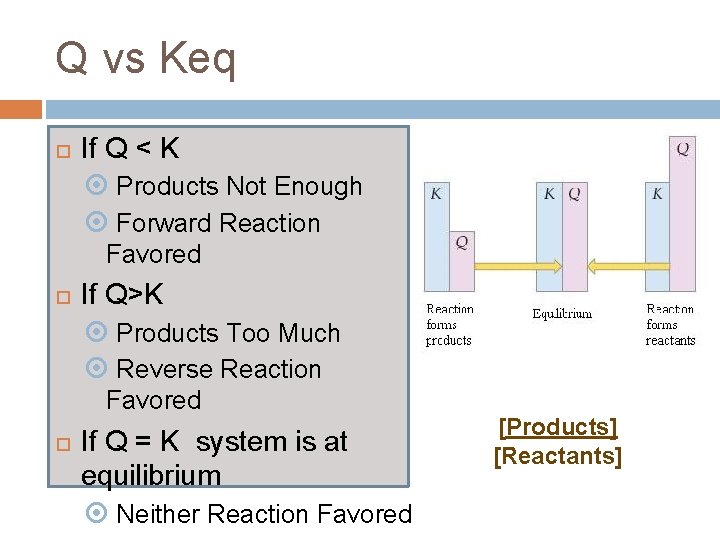
Q vs Keq If Q < K Products Not Enough Forward Reaction Favored If Q>K Products Too Much Reverse Reaction Favored If Q = K system is at equilibrium Neither Reaction Favored [Products] [Reactants]
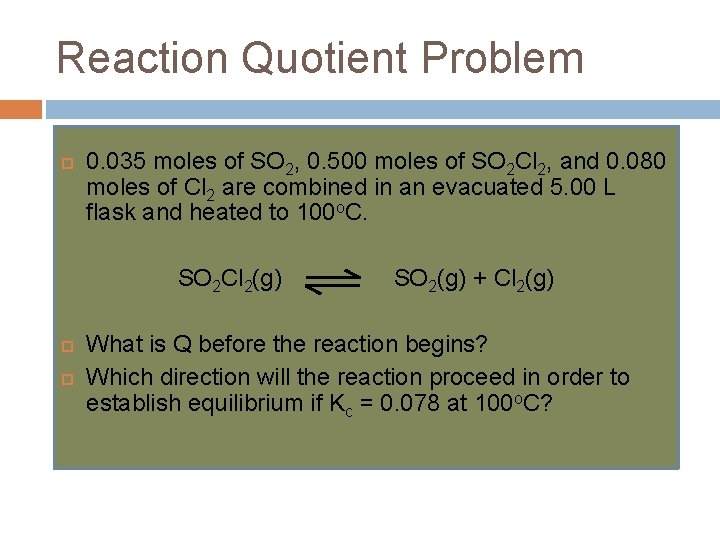
Reaction Quotient Problem 0. 035 moles of SO 2, 0. 500 moles of SO 2 Cl 2, and 0. 080 moles of Cl 2 are combined in an evacuated 5. 00 L flask and heated to 100 o. C. SO 2 Cl 2(g) SO 2(g) + Cl 2(g) What is Q before the reaction begins? Which direction will the reaction proceed in order to establish equilibrium if Kc = 0. 078 at 100 o. C?
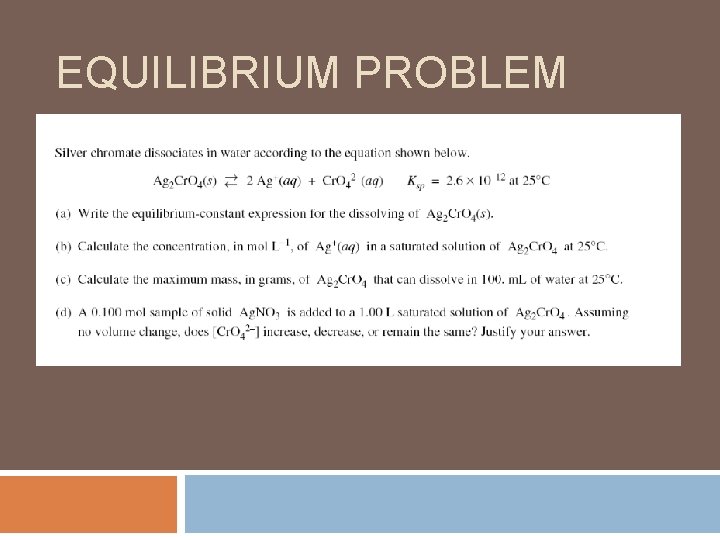
EQUILIBRIUM PROBLEM
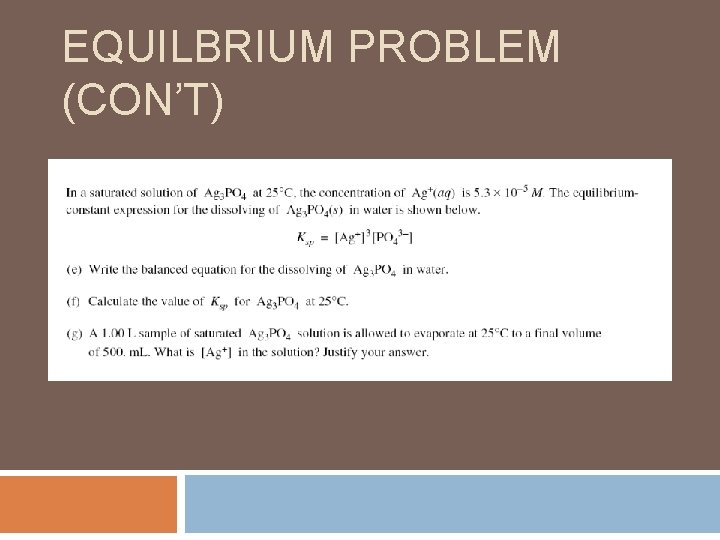
EQUILBRIUM PROBLEM (CON’T)

LE CHATELIER’S PRINCIPLE
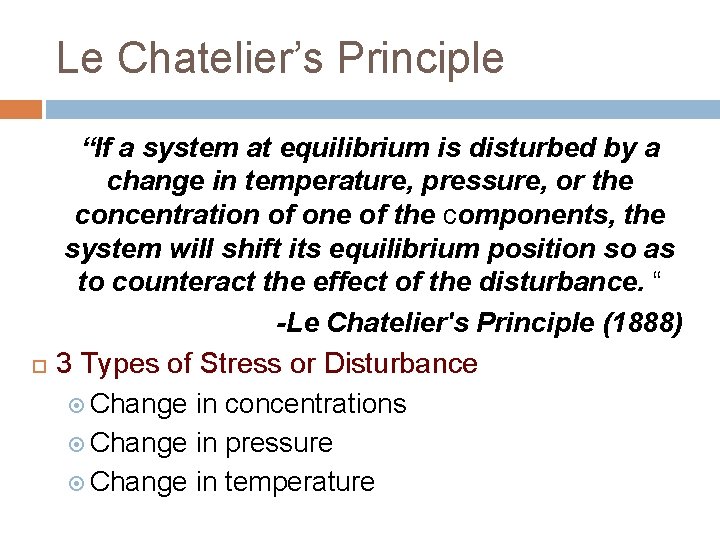
Le Chatelier’s Principle “If a system at equilibrium is disturbed by a change in temperature, pressure, or the concentration of one of the components, the system will shift its equilibrium position so as to counteract the effect of the disturbance. “ -Le Chatelier's Principle (1888) 3 Types of Stress or Disturbance Change in concentrations Change in pressure Change in temperature
Early Inspiration Fe 2 O 3(s) + 3 CO(g) 2 Fe(s) + 3 CO 2(g) The reduction of iron oxide by carbon monoxide The smoke emitted contained a lot of CO and iron production was slow. How could we remedy this? http: //www. youtube. com/watch? v=v. Wxs 7 ZV 5 Ly 8
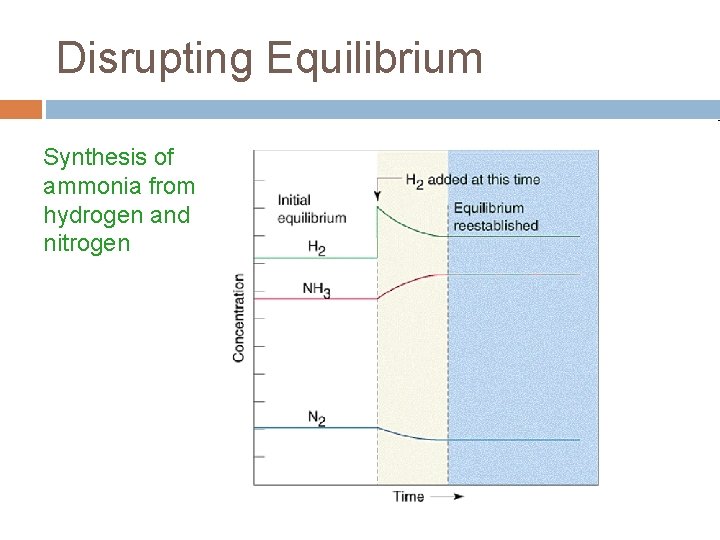
Disrupting Equilibrium Synthesis of ammonia from hydrogen and nitrogen
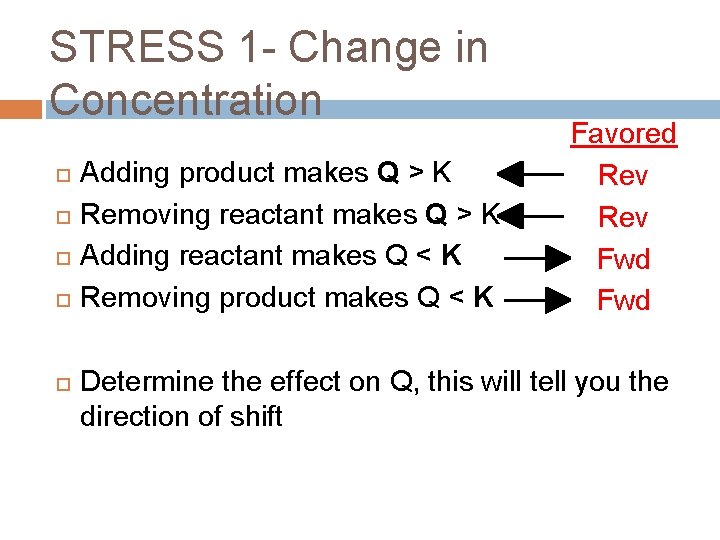
STRESS 1 - Change in Concentration Adding product makes Q > K Removing reactant makes Q > K Adding reactant makes Q < K Removing product makes Q < K Favored Rev Fwd Determine the effect on Q, this will tell you the direction of shift
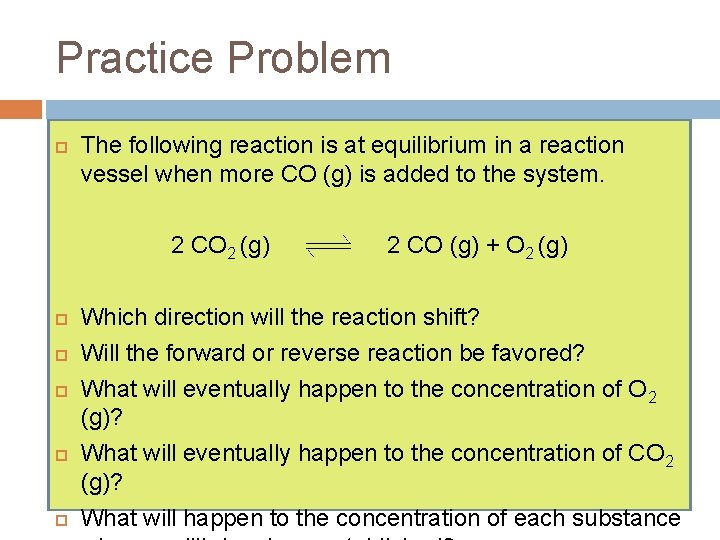
Practice Problem The following reaction is at equilibrium in a reaction vessel when more CO (g) is added to the system. 2 CO 2 (g) 2 CO (g) + O 2 (g) Which direction will the reaction shift? Will the forward or reverse reaction be favored? What will eventually happen to the concentration of O 2 (g)? What will eventually happen to the concentration of CO 2 (g)? What will happen to the concentration of each substance
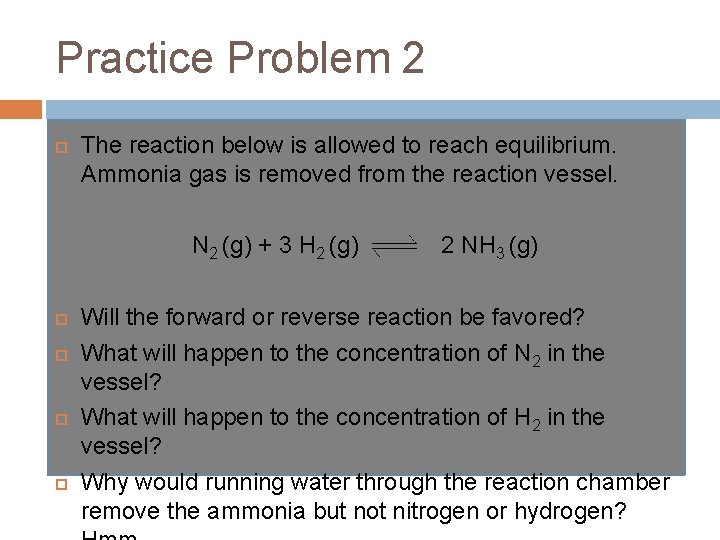
Practice Problem 2 The reaction below is allowed to reach equilibrium. Ammonia gas is removed from the reaction vessel. N 2 (g) + 3 H 2 (g) 2 NH 3 (g) Will the forward or reverse reaction be favored? What will happen to the concentration of N 2 in the vessel? What will happen to the concentration of H 2 in the vessel? Why would running water through the reaction chamber remove the ammonia but not nitrogen or hydrogen?

STRESS 2 - Change Pressure System will shift to minimize the total volume if pressure is increased. System will move in the direction that has the fewest moles of gas. If moles of gas molecules is equal, the system will not be affected. Because partial pressures (and concentrations) change a new equilibrium will be reached.
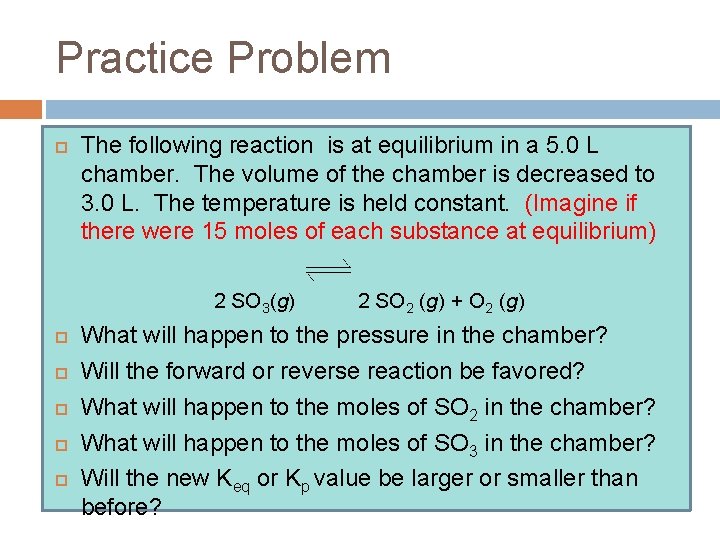
Practice Problem The following reaction is at equilibrium in a 5. 0 L chamber. The volume of the chamber is decreased to 3. 0 L. The temperature is held constant. (Imagine if there were 15 moles of each substance at equilibrium) 2 SO 3(g) 2 SO 2 (g) + O 2 (g) What will happen to the pressure in the chamber? Will the forward or reverse reaction be favored? What will happen to the moles of SO 2 in the chamber? What will happen to the moles of SO 3 in the chamber? Will the new Keq or Kp value be larger or smaller than before?

STRESS 3 - Change in Temperature Affects the rates of both the forward and reverse reactions – speeds up both reactions, so equilibrium is reached faster. The direction of the shift depends on whether the reaction is exothermic (- ∆H) or endothermic (+ ∆H) Think of heat as a product if exothermic: Reactant Product + Heat Think of heat as a reactant if endothermic: Reactant + Heat Product
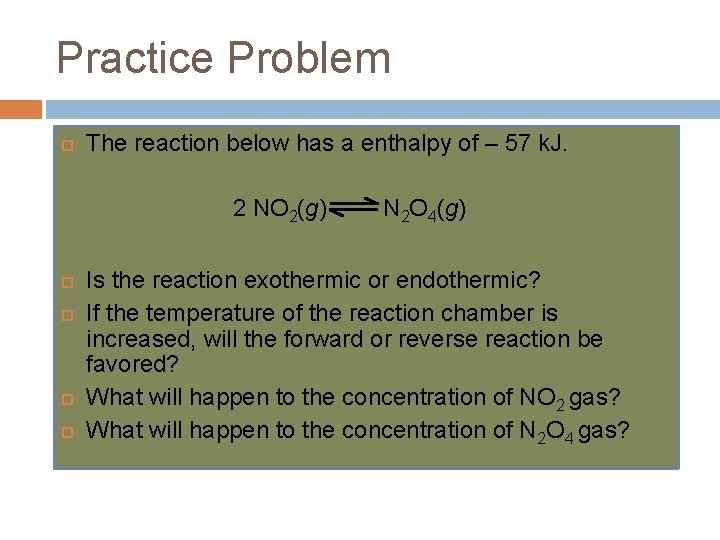
Practice Problem The reaction below has a enthalpy of – 57 k. J. 2 NO 2(g) N 2 O 4(g) Is the reaction exothermic or endothermic? If the temperature of the reaction chamber is increased, will the forward or reverse reaction be favored? What will happen to the concentration of NO 2 gas? What will happen to the concentration of N 2 O 4 gas?
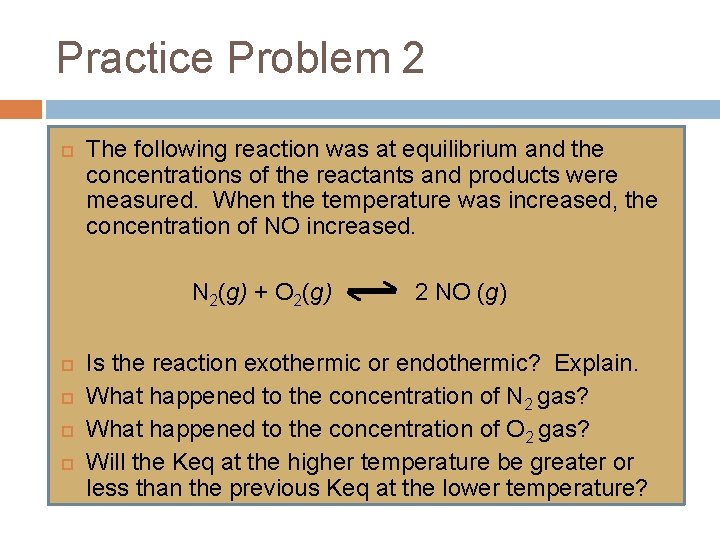
Practice Problem 2 The following reaction was at equilibrium and the concentrations of the reactants and products were measured. When the temperature was increased, the concentration of NO increased. N 2(g) + O 2(g) 2 NO (g) Is the reaction exothermic or endothermic? Explain. What happened to the concentration of N 2 gas? What happened to the concentration of O 2 gas? Will the Keq at the higher temperature be greater or less than the previous Keq at the lower temperature?
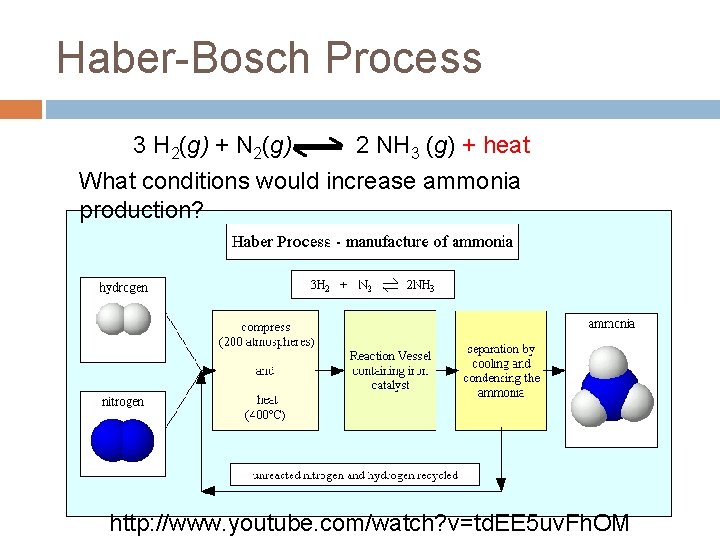
Haber-Bosch Process 3 H 2(g) + N 2(g) 2 NH 3 (g) + heat What conditions would increase ammonia production? http: //www. youtube. com/watch? v=td. EE 5 uv. Fh. OM
- Slides: 20