SOLVENT EXTRACTION AND SOLID PHASE EXTRACTION University of







![EXTRACTION EQUILIBRIA OF CHELATE EXTRACTION SYSTEM HRaq HRorg KDR H+ + R - [HR]O EXTRACTION EQUILIBRIA OF CHELATE EXTRACTION SYSTEM HRaq HRorg KDR H+ + R - [HR]O](https://slidetodoc.com/presentation_image_h2/0ca6c0c54ab768dd3154da534c9e5a98/image-10.jpg)
![D= [M]o [MRn]O ………… 5 = [M]n+ D= Kf KDXKa [HR]on KDRn [H+]n . D= [M]o [MRn]O ………… 5 = [M]n+ D= Kf KDXKa [HR]on KDRn [H+]n .](https://slidetodoc.com/presentation_image_h2/0ca6c0c54ab768dd3154da534c9e5a98/image-11.jpg)


![High Molecular Weight Mass Amines Ø Tertiary Amine Trioctyl amine N – [CH 2]7 High Molecular Weight Mass Amines Ø Tertiary Amine Trioctyl amine N – [CH 2]7](https://slidetodoc.com/presentation_image_h2/0ca6c0c54ab768dd3154da534c9e5a98/image-18.jpg)






- Slides: 29

SOLVENT EXTRACTION AND SOLID PHASE EXTRACTION University of Mumbai Dept. of Chemistry M. Sc(I), Semester (I) Faculty: Dr. Manjusha Karve

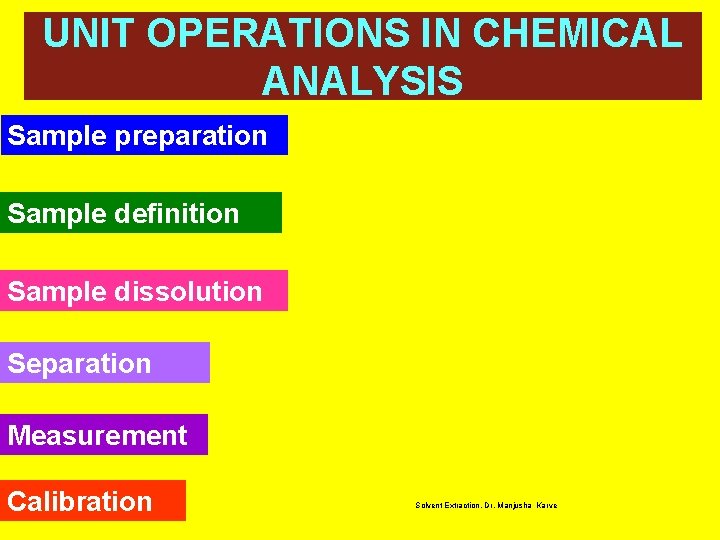
UNIT OPERATIONS IN CHEMICAL ANALYSIS Sample preparation Sample definition Sample dissolution Separation Measurement Calibration Solvent Extraction, Dr. Manjusha Karve

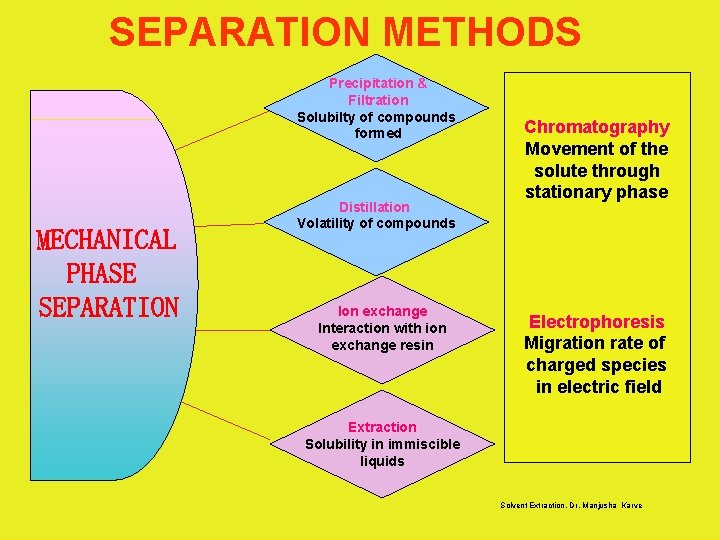
SEPARATION METHODS Precipitation & Filtration Solubilty of compounds formed MECHANICAL PHASE SEPARATION Distillation Volatility of compounds Ion exchange Interaction with ion exchange resin Chromatography Movement of the solute through stationary phase Electrophoresis Migration rate of charged species in electric field Extraction Solubility in immiscible liquids Solvent Extraction, Dr. Manjusha Karve

SOLVENT EXTRACTION A popular technique for separation and determination of metals based on relative solubility of an analyte in two immiscible liquids. Simple and selective. Preconcentrate species prior to analysis. Applicable at trace as well as macro levels. Solvent Extraction, Dr. Manjusha Karve

PRINCIPLES OF SOLVENT EXTRACTION Distribution law Zaq Zorg Phase Rule P+F = C+2 Where, P = No. of phases, C = No. of components, F = No. of degrees of freedom According to Nernst Distribution Law at constant temperature and pressure, Distribution Coefficient [Z ] KD = [Z o. ] aq. Distribution ratio D = %E= Total conc. of all forms of Z in organic phase Total conc. of all forms of Z in aqueous phase 100 D D(V/VO) Solvent Extraction, Dr. Manjusha Karve

PROCESS OF SOLVENT EXTRACTION Pre requisite of solvent extraction 1. Formation of neutral or uncharged species 2. Chemical reaction in aqueous phase to form uncharged or zero valent species 3. Distribution of extractable complete 4. Chemical interaction in organic phase Solvent Extraction, Dr. Manjusha Karve

CLASSIFICATION OF SOLVENT EXTRACTION SYSTEMS ØExtraction by Chelation Dimethylglyoxime Ni 2+ Chloroform ØExtraction by Solvation Fe 3+ HCl [6 M] [H(Fe. Cl)4] Ethyl acetate ØExtraction by Ion Pair Formation Tetraphenylarsonium [(C 6 H 5)4 As+] Perrhenate Re. O 4 - ØSynnergistic Extraction Solvent Extraction, Dr. Manjusha Karve

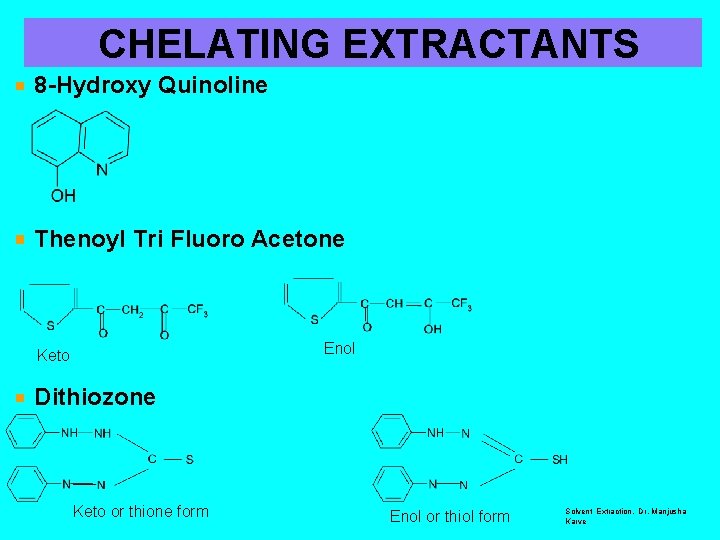
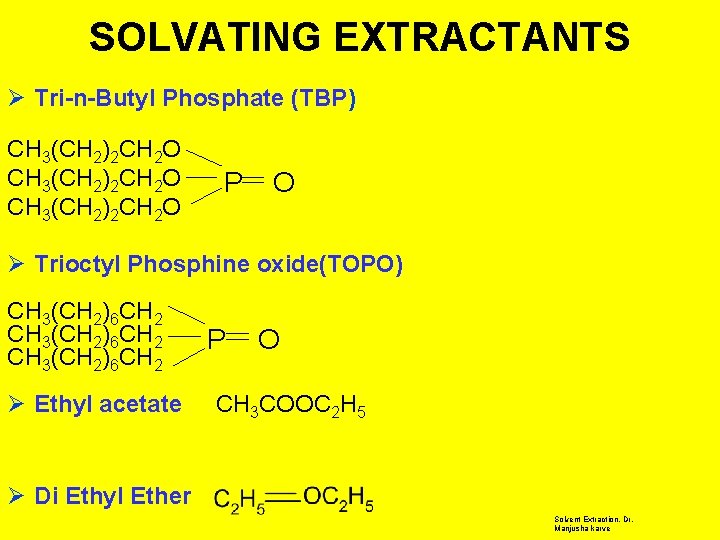
TYPES OF EXTRACTANTS Ø § § § CHELATING EXTRACTANTS 8 -Hydroxy Quinoline Thenoyltrifluoroacetone Dithizone Ø § § SOLVATING EXTRACTANTS Tributyl phosphate Trioctylphosphineoxide Ethylacetate Ether Ø CROWN ETHERS Ø LIQUID ANION & CATION EXCHANGERS Solvent Extraction, Dr. Manjusha Karve

CHELATE SYSTEM EXTRACTION EQUILIBRIA HR HR MRn H + + R - + M n+ MRn Solvent Extraction, Dr Manjusha Karve
![EXTRACTION EQUILIBRIA OF CHELATE EXTRACTION SYSTEM HRaq HRorg KDR H R HRO EXTRACTION EQUILIBRIA OF CHELATE EXTRACTION SYSTEM HRaq HRorg KDR H+ + R - [HR]O](https://slidetodoc.com/presentation_image_h2/0ca6c0c54ab768dd3154da534c9e5a98/image-10.jpg)
EXTRACTION EQUILIBRIA OF CHELATE EXTRACTION SYSTEM HRaq HRorg KDR H+ + R - [HR]O = [HR] Ka [H+] [R-] ……… 2 = [HR] Mn+ + n. HR MRn + n. H+ Mn+ + n. R- MRn [MRn]org ……… 1 [MRn] ……… 3 [Mn+] [R-]n [MRn]O ……… 4 = [MRn] Kf = KDX Solvent Extraction, Dr. Manjusha Karve
![D Mo MRnO 5 Mn D Kf KDXKa HRon KDRn Hn D= [M]o [MRn]O ………… 5 = [M]n+ D= Kf KDXKa [HR]on KDRn [H+]n .](https://slidetodoc.com/presentation_image_h2/0ca6c0c54ab768dd3154da534c9e5a98/image-11.jpg)
D= [M]o [MRn]O ………… 5 = [M]n+ D= Kf KDXKa [HR]on KDRn [H+]n . ………. 6 D = Distribution ratio of the analyte KDR = Distribution constant of the chelating reagent KDX = Distribution constant of the extracted species Ka = Acid Dissociation constant of the chelating reagent Kf = Formation constant of the metal complex • Solvent Extraction, Dr. Manjusha Karve

CHELATING EXTRACTANTS 8 -Hydroxy Quinoline Thenoyl Tri Fluoro Acetone Enol Keto Dithiozone Keto or thione form Enol or thiol form Solvent Extraction, Dr. Manjusha Karve

SOLVATING EXTRACTANTS Ø Tri-n-Butyl Phosphate (TBP) CH 3(CH 2)2 CH 2 O P O Ø Trioctyl Phosphine oxide(TOPO) CH 3(CH 2)6 CH 2 Ø Ethyl acetate P O CH 3 COOC 2 H 5 Ø Di Ethyl Ether Solvent Extraction, Dr. Manjusha karve

Macro cyclic poly ethers Central Hydrophilic cavity diameter 1. 26. 0 A° Central Cavity – Ringed with atoms like Oxygen, Sulphur and Nitrogen These atoms surrounded by –CH 2 groups Solvent Extraction, Dr. Manjusha Karve

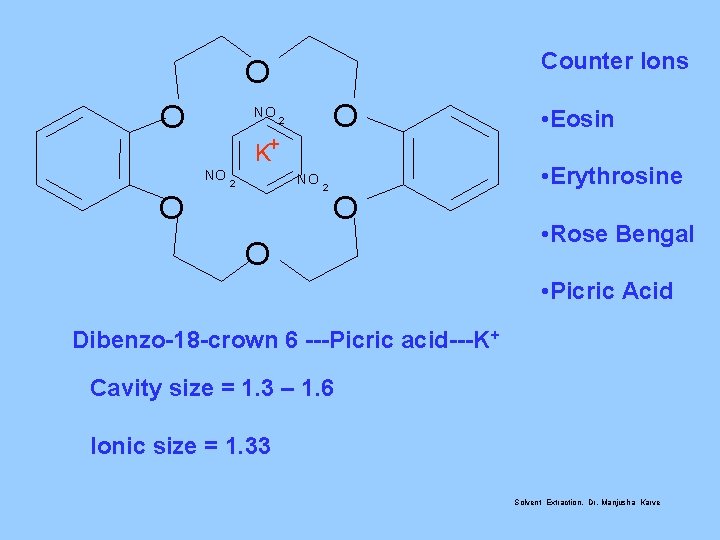
Counter Ions O O NO O 2 K+ NO NO 2 • Eosin • Erythrosine O O • Rose Bengal • Picric Acid Dibenzo-18 -crown 6 ---Picric acid---K+ Cavity size = 1. 3 – 1. 6 Ionic size = 1. 33 Solvent Extraction, Dr. Manjusha Karve

LIQUID ION EXCHANGERS • LIQUID ANION EXCHANGERS – • WEAK ORGANOPHILIC BASES • MECHANISM OF EXTRACTION- ANION EXCHANGE • LIQUID CATION EXCHANGERS – • ORGANO PHOSPHORUS ACIDS • MECHANISM OF EXTRACTION – CATION EXCHANGE Solvent Extraction, Dr. Manjusha Karve

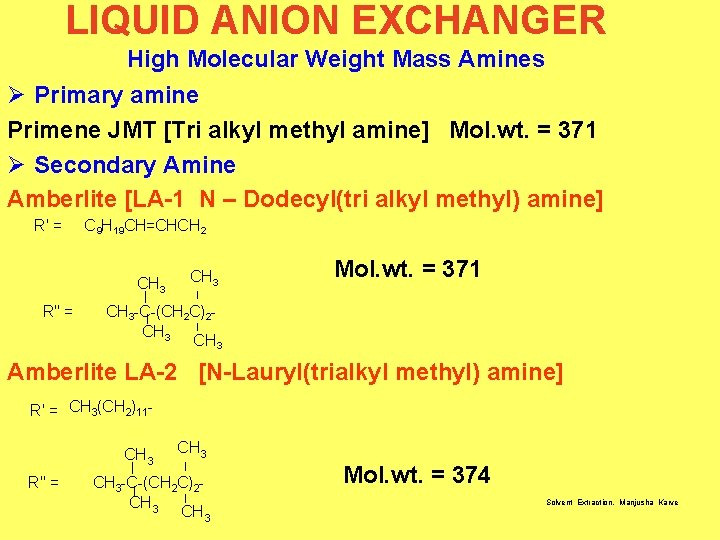
LIQUID ANION EXCHANGER High Molecular Weight Mass Amines Ø Primary amine Primene JMT [Tri alkyl methyl amine] Mol. wt. = 371 Ø Secondary Amine Amberlite [LA-1 N – Dodecyl(tri alkyl methyl) amine] R' = C 9 H 19 CH=CHCH 2 CH 3 R" = CH 3 Mol. wt. = 371 CH 3 -C-(CH 2 C) 2 CH 3 Amberlite LA-2 [N-Lauryl(trialkyl methyl) amine] R' = CH 3(CH 2) 11 CH 3 R" = CH 3 -C-(CH 2 C) 2 CH 3 Mol. wt. = 374 Solvent Extraction, Manjusha Karve
![High Molecular Weight Mass Amines Ø Tertiary Amine Trioctyl amine N CH 27 High Molecular Weight Mass Amines Ø Tertiary Amine Trioctyl amine N – [CH 2]7](https://slidetodoc.com/presentation_image_h2/0ca6c0c54ab768dd3154da534c9e5a98/image-18.jpg)
High Molecular Weight Mass Amines Ø Tertiary Amine Trioctyl amine N – [CH 2]7 -CH 3]3 Mol. wt. = 353 Ø Quaternary Amine Aliquat 336 S [Tri alkyl Methyl ammonium chloride] [CH 3 -N-(-CH 2)7 -11(CH 3)3]Cl Mol. wt. = 475 Solvent Extraction, Dr. Manjusha Karve

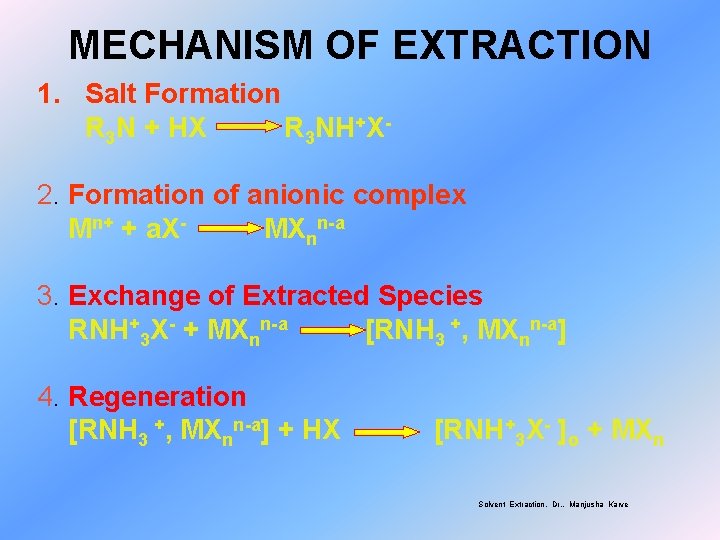
MECHANISM OF EXTRACTION 1. Salt Formation R 3 N + HX R 3 NH+X 2. Formation of anionic complex Mn+ + a. XMXnn-a 3. Exchange of Extracted Species RNH+3 X- + MXnn-a [RNH 3 +, MXnn-a] 4. Regeneration [RNH 3 +, MXnn-a] + HX [RNH+3 X- ]o + MXn Solvent Extraction, Dr. . Manjusha Karve

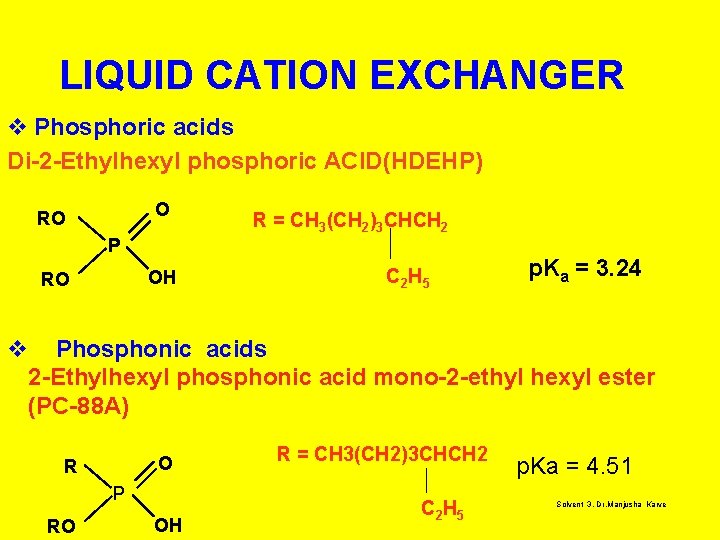
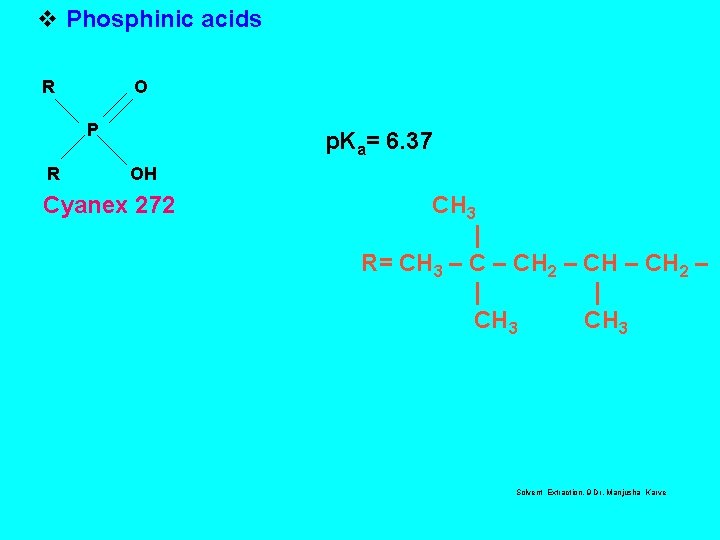
LIQUID CATION EXCHANGER v Phosphoric acids Di-2 -Ethylhexyl phosphoric ACID(HDEHP) O RO R = CH 3(CH 2)3 CHCH 2 P OH RO C 2 H 5 p. Ka = 3. 24 v Phosphonic acids 2 -Ethylhexyl phosphonic acid mono-2 -ethyl hexyl ester (PC-88 A) O R P RO OH R = CH 3(CH 2)3 CHCH 2 C 2 H 5 p. Ka = 4. 51 Solvent 3, Dr. Manjusha Karve

v Phosphinic acids R O P R p. Ka= 6. 37 OH Cyanex 272 CH 3 | R= CH 3 – CH 2 – | | CH 3 Solvent Extraction, 9 Dr. Manjusha Karve

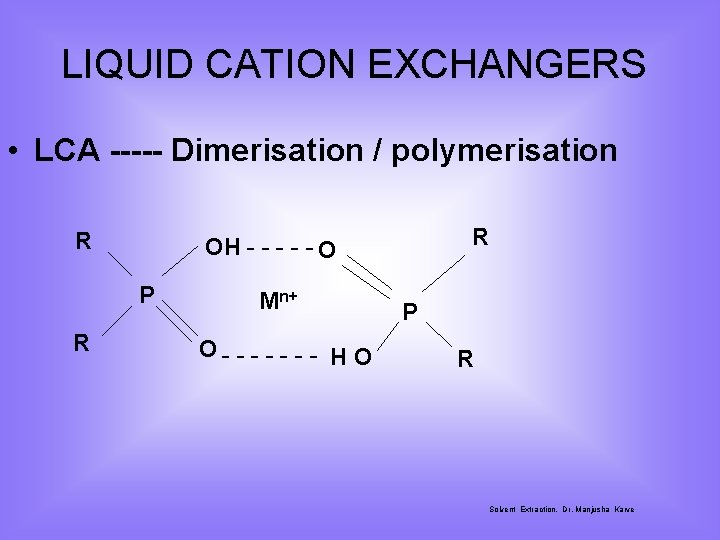
LIQUID CATION EXCHANGERS • LCA ----- Dimerisation / polymerisation R P R R OH - - - O Mn+ O- - - - H O P R Solvent Extraction, Dr. Manjusha Karve

APPLICATIONS OF SOLVENT EXTRACTION Ø Real Samples Ø Water analysis Ø Soil analysis Ø Ores and Alloys Solvent Extraction, Dr Manjusha Karve

NEW SEPARATION METHODS • • Selective Rapid Enrichment factor Detection at ppb / ppt level Solvent Extraction, Dr. Manjusha Karve

WHY SOLID PHASE EXTRACTION ØSolvent Extraction v. Merits Simplicity, rapidity, ready adaptability of scale up studies and easier recovery of metal v. Demerits Labor intensive procedure, use of relatively large volumes of solvents, need of high purity solvents, relatively low preconcentration factor and disposal of waste generated is an environmental concern Solvent Extraction, Dr. Manjusha Karve

SPE v. Minimizes use of organic solvents v. Economical vlow cost for solvents and waste diposal v. Absence of emulsion formation v. Environmentally friendly v. High preconcentration / enrichment factor v. Sample clean up v. Easy automation Solvent Extraction, Dr. Manjusha Karve

Ø Transfer of analytes from the aqueous phase Ø Active sites of solid phase ØSolid-liquid extraction and extraction chromatography Ø Three major components Liquid phase ---- Target analyte ----- sorbent ØOPTIMISATION OF EXTRACTION CONDITIONS Analyte of interest, nature of sorbent, time of contact between the three, p. H and other aqueous phase parameters Solvent Extraction, Dr. Manjusha Karve

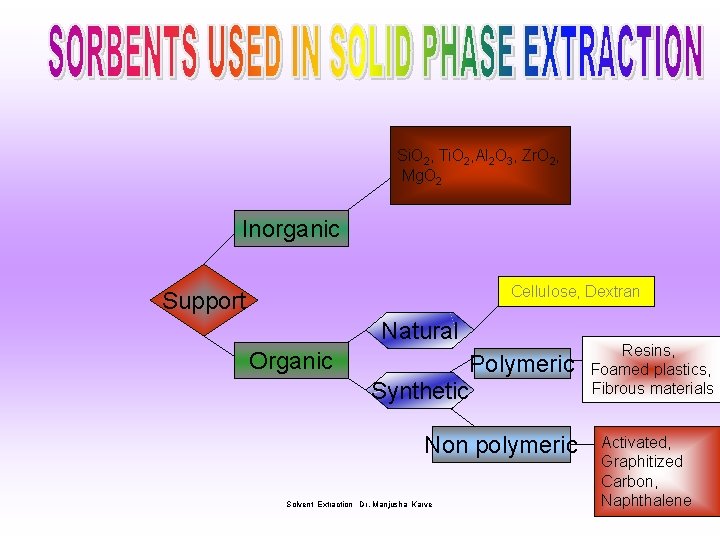
Si. O 2, Ti. O 2, Al 2 O 3, Zr. O 2, Mg. O 2 Inorganic Cellulose, Dextran Support Natural Organic Polymeric Synthetic Non polymeric Solvent Extraction Dr. Manjusha Karve Resins, Foamed plastics, Fibrous materials Activated, Graphitized Carbon, Naphthalene

References 1. 2. 3. 4. 5. G. D Christian, Analytical Chemistry, Sixth edition, John Wiley and Sons, New York (2004) J. Basset, R. C. Denney, G. H. Jeffery, J. Mendham, Vogel’s Textbook of Quantitative Chemical Analysis, ELBS, Longman Scientific and Technical, England (2002) S. M. Khopkar, Instrumental Methods of Bioanalytical Chemistry, New Age International Publishers (2016) D. A. Skoog, D. M. West, F. J. Holler, S. R. Crouch, Fundamentals of Analytical Chemistry, Eighth edition, Philadelphia, Saunders College Publishing (2004) Solid Phase Extraction- Principles, Techniques and Applications, N. J. K. Simpson, Marcel Dekker, New York (2004) Solvent Extraction, Dr. Manjusha Karve