SOLUTIONS When two substances are mixed 1 as

SOLUTIONS When two substances are mixed: 1) as a result of a chemical reaction a new substance formed 2) there no chemical reaction and only a mechanical mixture formed 3) the forming of a solution of as a result of a chemical reaction

• Solution - is a homogeneous system consisting of two or more components • The composition of a solution can vary within certain limits without changing homogeneity • A solvent is a component solution and its concentration is higher than other components • The solvent does not change its phase when a solution is formed

• True solutions have the size of particles (molecules) equaled 1 nm. This is thermodynamically stable single-phase multi-component systems • Colloidal solutions have a particle size from 1 to 100 nm. There exist, if the particles have a charge. • Disperse systems is colloidal solutions when charges on the particles absent. A particle size is more than 100 nm.
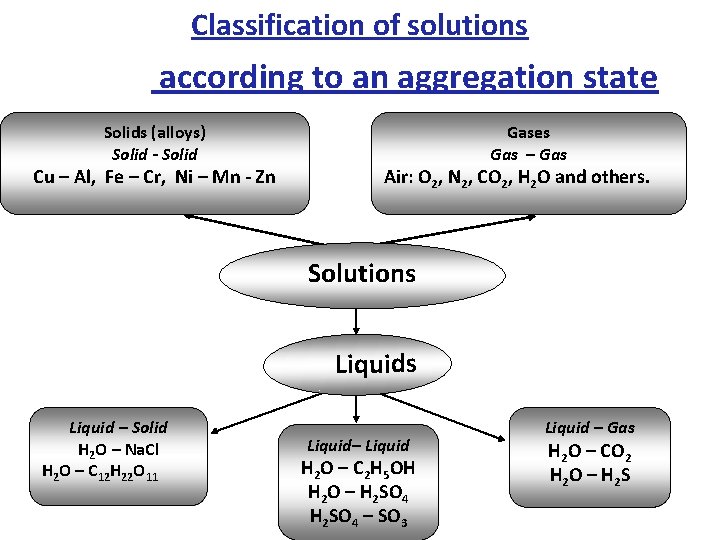
Classification of solutions according to an aggregation state Solids (alloys) Solid - Solid Cu – Al, Fe – Cr, Ni – Mn - Zn Gases Gas – Gas Air: O 2, N 2, CO 2, H 2 O and others. Solutions Liquid – Solid H 2 O – Na. Cl H 2 O – C 12 H 22 O 11 Liquid– Liquid H 2 O – C 2 H 5 OH H 2 O – H 2 SO 4 – SO 3 Liquid – Gas H 2 O – CO 2 H 2 O – H 2 S

Classification of solutions according to electrical conductivity: • solutions - electrolytes • solutions – nonelectrolytes according to the type of a solvent: • solvent - water • solvent - ammonia, benzene, carbon tetrachloride, dichloroethane, etc.
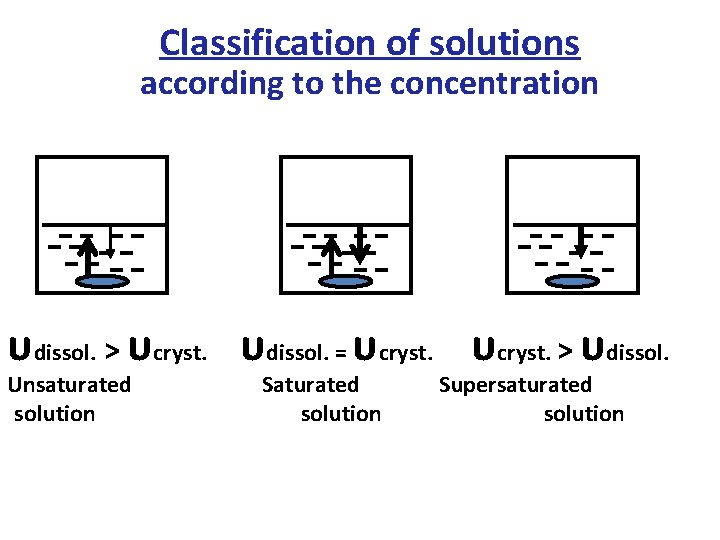
Classification of solutions according to the concentration υdissol. > υcryst. υdissol. = υcryst. > υdissol. Unsaturated solution Supersaturated solution
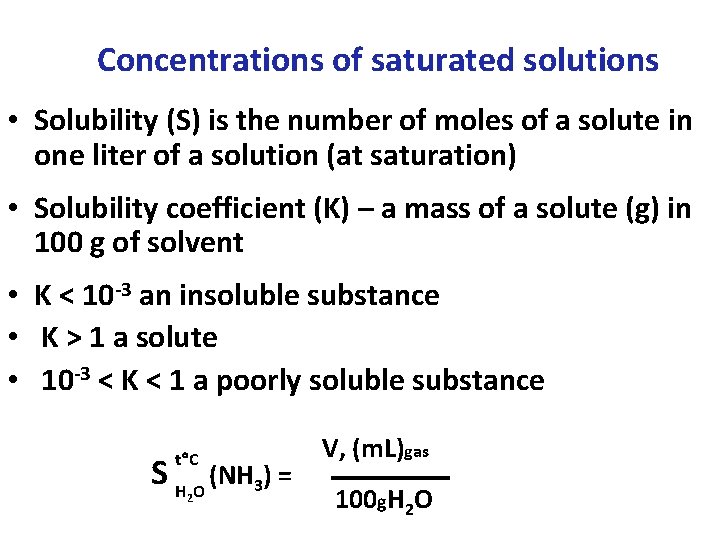
Concentrations of saturated solutions • Solubility (S) is the number of moles of a solute in one liter of a solution (at saturation) • Solubility coefficient (K) – a mass of a solute (g) in 100 g of solvent • K < 10 -3 an insoluble substance • K > 1 a solute • 10 -3 < K < 1 a poorly soluble substance t С S H O (NH 3) = 2 V, (m. L)gas 100 g. Н 2 О
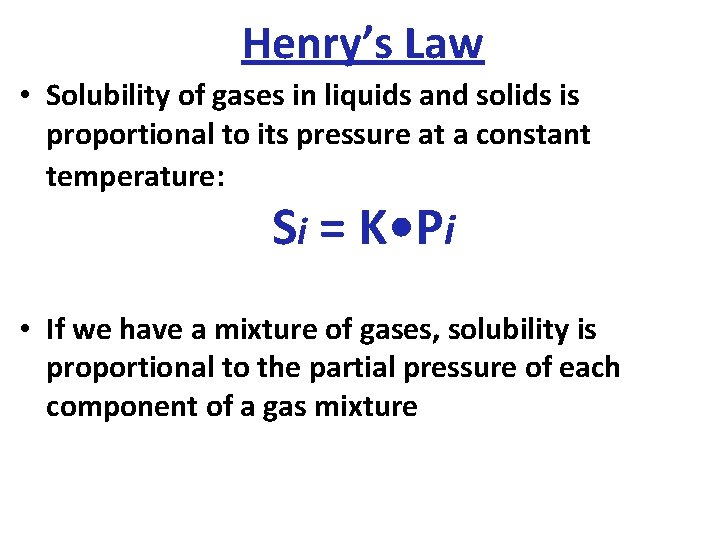
Henry’s Law • Solubility of gases in liquids and solids is proportional to its pressure at a constant temperature: Si = K • Pi • If we have a mixture of gases, solubility is proportional to the partial pressure of each component of a gas mixture

Concentrations of unsaturated solutions • The concentration of an unsaturated solution is characterized by the amount of a solute per an unit of weight or volume of a solution
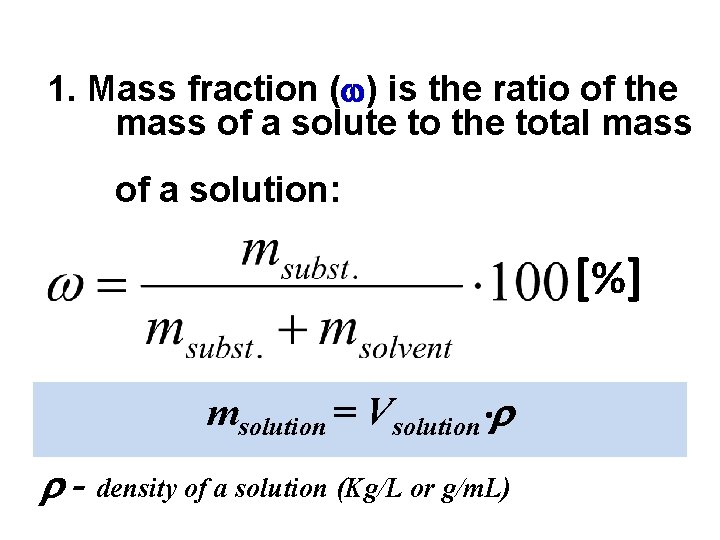
1. Mass fraction ( ) is the ratio of the mass of a solute to the total mass of a solution: [ %] msolution = Vsolution - density of a solution (Kg/L or g/m. L)
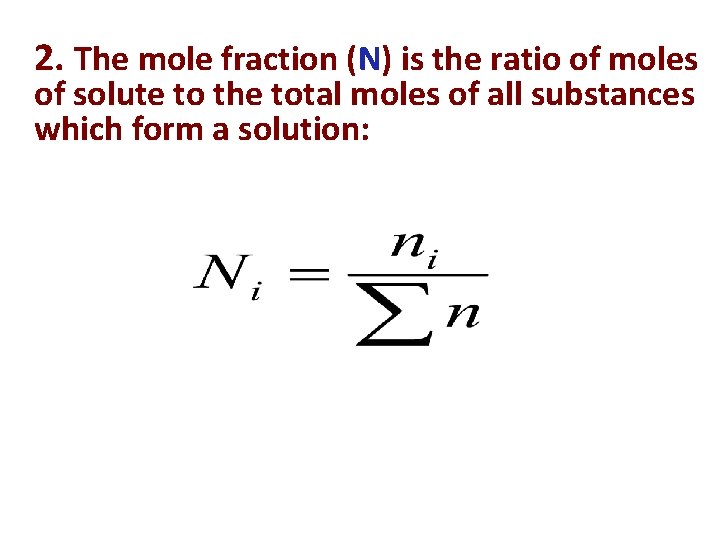
2. The mole fraction (N) is the ratio of moles of solute to the total moles of all substances which form a solution:
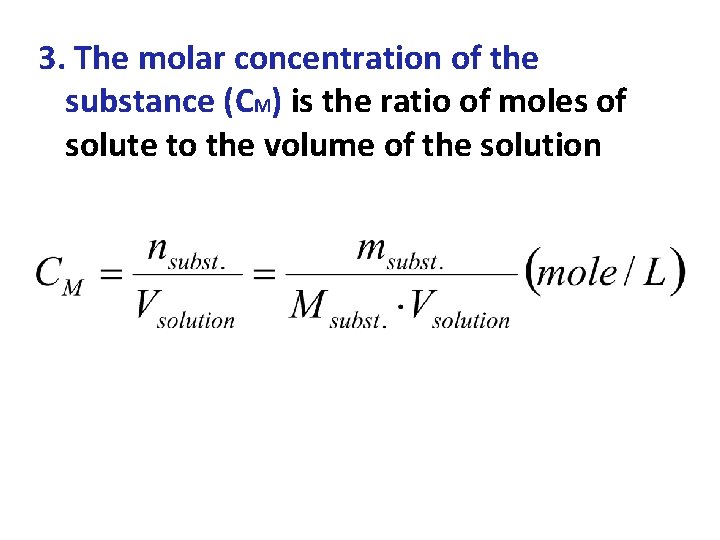
3. The molar concentration of the substance (CM) is the ratio of moles of solute to the volume of the solution
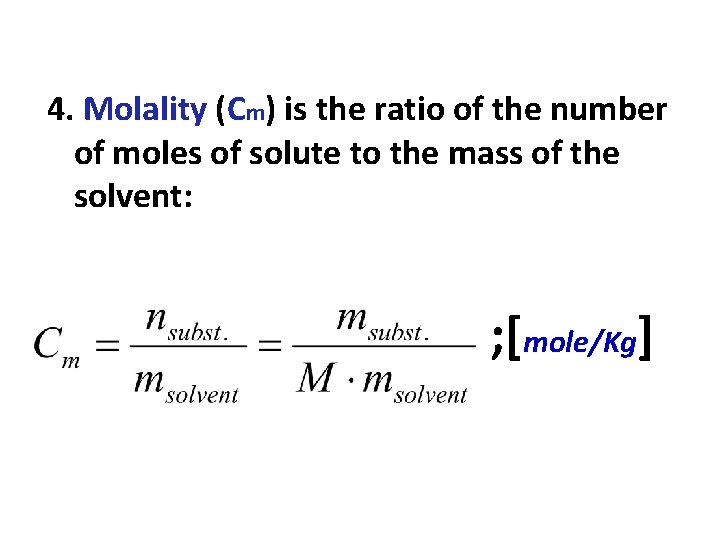
4. Molality (Cm) is the ratio of the number of moles of solute to the mass of the solvent: ; [mole/Kg]
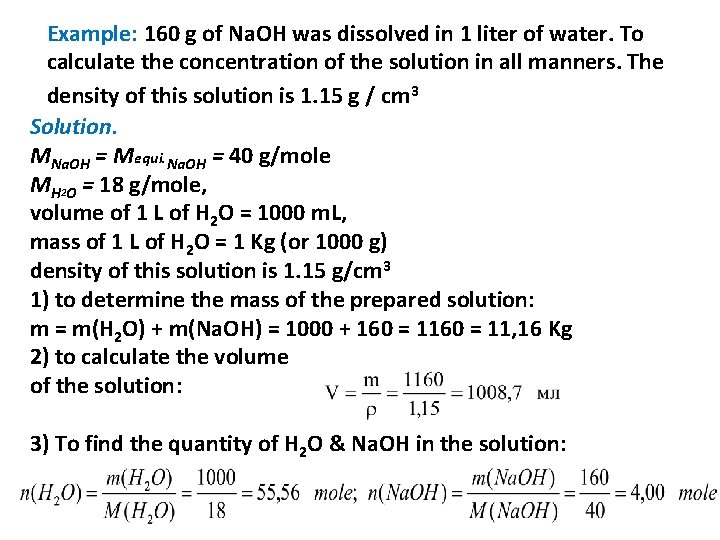
Example: 160 g of Na. OH was dissolved in 1 liter of water. To calculate the concentration of the solution in all manners. The density of this solution is 1. 15 g / cm 3 Solution. MNa. OH = Mequi. Na. OH = 40 g/mole MH 2 O = 18 g/mole, volume of 1 L of H 2 O = 1000 m. L, mass of 1 L of H 2 O = 1 Kg (or 1000 g) density of this solution is 1. 15 g/cm 3 1) to determine the mass of the prepared solution: m = m(H 2 O) + m(Na. OH) = 1000 + 160 = 11, 16 Kg 2) to calculate the volume of the solution: 3) To find the quantity of H 2 O & Na. OH in the solution:
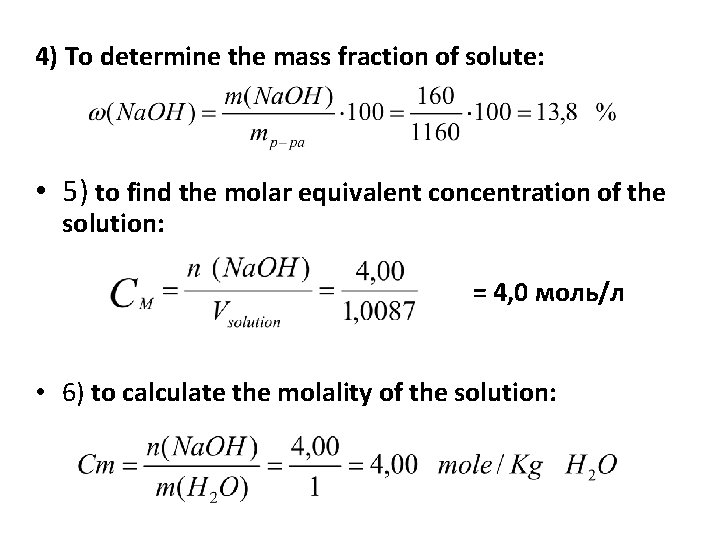
4) To determine the mass fraction of solute: • 5) to find the molar equivalent concentration of the solution: = 4, 0 моль/л • 6) to calculate the molality of the solution:
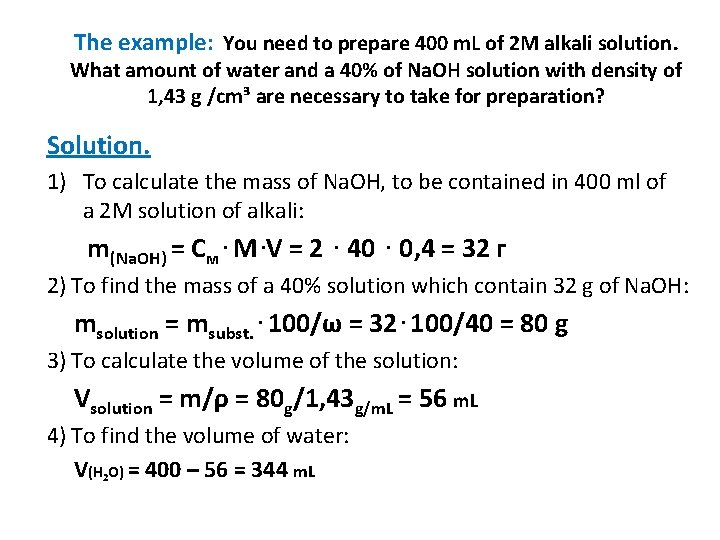
The example: You need to prepare 400 m. L of 2 M alkali solution. What amount of water and a 40% of Na. OH solution with density of 1, 43 g /cm³ are necessary to take for preparation? Solution. 1) To calculate the mass of Na. OH, to be contained in 400 ml of a 2 M solution of alkali: m(Na. OH) = См. M. V = 2. 40. 0, 4 = 32 г 2) To find the mass of a 40% solution which contain 32 g of Na. OH: msolution = msubst. . 100/ω = 32. 100/40 = 80 g 3) To calculate the volume of the solution: Vsolution = m/ρ = 80 g/1, 43 g/m. L = 56 m. L 4) To find the volume of water: V(H 2 O) = 400 – 56 = 344 m. L

Factors affecting on the rate of dissolution • The dissolution rate is maximum at the beginning and gradually decreases to zero • • A powdery substance is dissolved at a greater rate because a surface increases • The dissolution rate increases with heating and stirring, as Kd increased

Solutions of Nonelectrolytes

Properties of Dilute Solutions of Non-electrolytes Ø Non-electrolyte solution is similar the ideal solution according to the properties. It means that molecules are connected with weak intermolecular forces. Ø Their properties do not depend on the nature of the solute and they depend on the quantity of the particles. Such properties are called the colligative properties. Ø The dissolution process is ( G 0) due to the increase of entropy (-Т S)

Colligative Properties of Non-electrolytes Solutions Ø The saturated vapor pressure of the solvent above a solution Ø Boiling point Ø Freezing point Ø Osmotic pressure

Ø At any temperature there is evaporation of a liquid. Ø In a closed vessel condensation, as a reverse process, goes simultaneously with the evaporation. Ø At equilibrium, when the rates of an evaporation is equal a condensation, steam above the liquid is called saturated. Ø The saturated vapor pressure is a characteristic of a liquid volatility: the higher the pressure, the more volatile is.

For Example: Vapor pressure at 20⁰C Ø above water - 2337 Pa, Ø above mercury - 0, 173 Pa, Ø above alcohol - 5866 Pa Ø above acetone - 24227 Pa. Volatility of Acetone> Alcohol> Water> Mercury On heating the pressure of a saturated vapor above a liquid increases
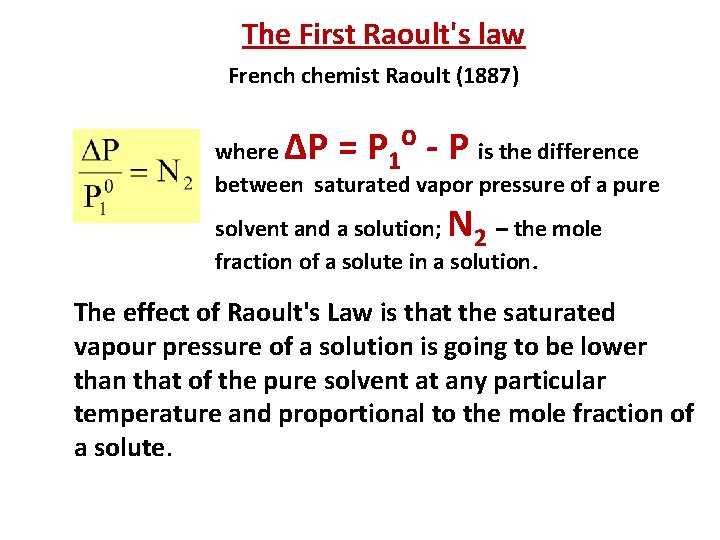
The First Raoult's law French chemist Raoult (1887) ∆Р = P ⁰ - P where is the difference 1 between saturated vapor pressure of a pure N solvent and a solution; 2 – the mole fraction of a solute in a solution. The effect of Raoult's Law is that the saturated vapour pressure of a solution is going to be lower than that of the pure solvent at any particular temperature and proportional to the mole fraction of a solute.

Problem Calculate the vapor pressure over 4% of the aqueous solution of glucose (C 6 H 12 O 6 ) at 40⁰C. P⁰ 1 = 2333 Pa.
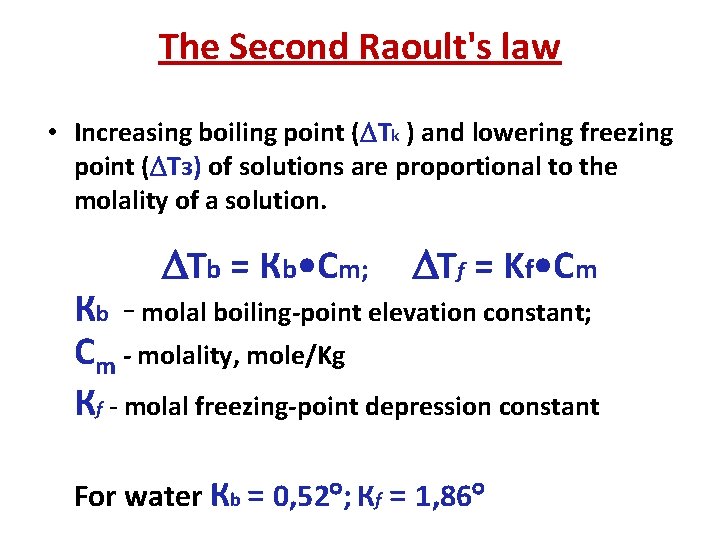
The Second Raoult's law • Increasing boiling point ( Tk ) and lowering freezing point ( Tз) of solutions are proportional to the molality of a solution. Tb = Кb • Cm; Tf = Kf • Cm Кb - molal boiling-point elevation constant; Сm - molality, mole/Kg Кf - molal freezing-point depression constant For water Кb = 0, 52 ; Кf = 1, 86

Problem A solution contains 3. 2 g of sulfur in 40 g of benzene, which has boiling point 80, 90 C. Pure benzene boils at 80. 10 C. Determine the number of atoms in a molecule of sulfur.

Problem A liquid, consisting of 8 liters of water and 2 liters of methyl alcohol ( = 0, 8 g /ml), was poured in a car radiator. What is the lowest temperature when the car can be left in the open air and the liquid in the radiator will not freeze?
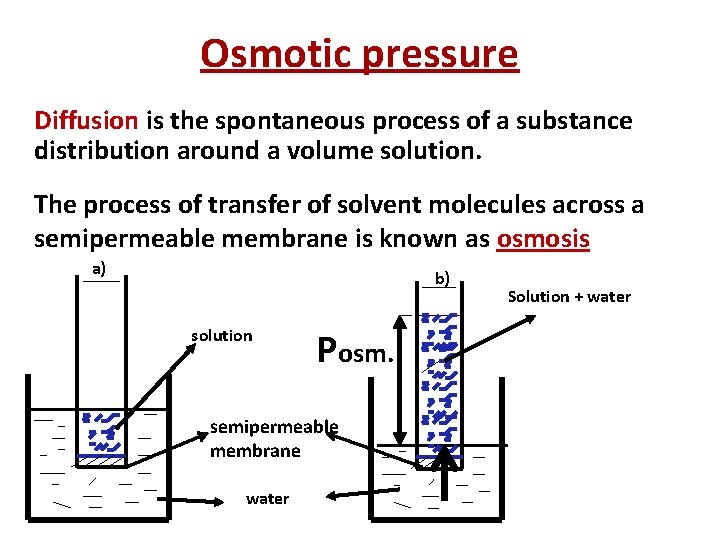
Osmotic pressure Diffusion is the spontaneous process of a substance distribution around a volume solution. The process of transfer of solvent molecules across a semipermeable membrane is known as osmosis a) b) solution Posm. semipermeable membrane water Solution + water
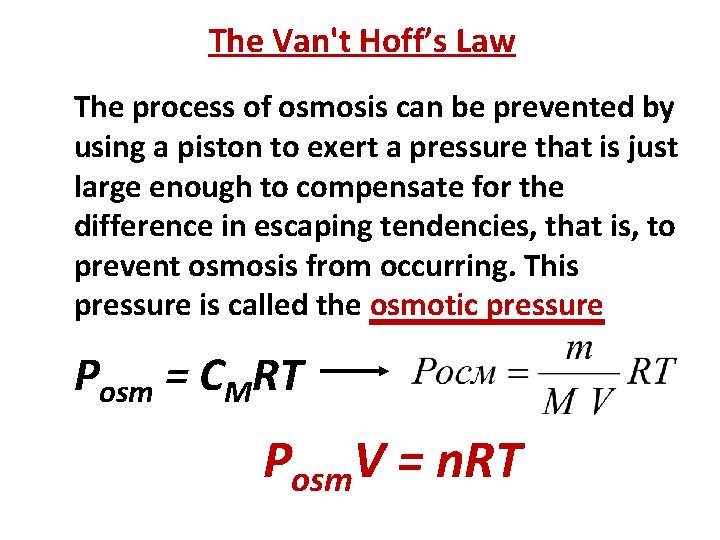
The Van't Hoff’s Law The process of osmosis can be prevented by using a piston to exert a pressure that is just large enough to compensate for the difference in escaping tendencies, that is, to prevent osmosis from occurring. This pressure is called the osmotic pressure Posm = CMRT Posm. V = n. RT

Problem There is 500 m. L of a solution containing 17. 1 g of sugar, С 12 H 22 O 11. Determine the osmotic pressure of this solution at 17 C.

Electrolyte Solutions

Electrolyte Solutions • These solutions had a greater conductivity than the pure solvent • A substance dissociates into ions when it dissolves. • In a solution the ions are hydrated or solvated. • Very dilute solutions of electrolytes are close to ideal solutions. In this case it is necessary to consider only the number of the formed particles in a solution. • In the concentrated solutions there are strong interaction of ions with formation of complex particles and reducing the total number of particles. • The degree of dissociation of solute ions depend on the nature of the solute and solvent.
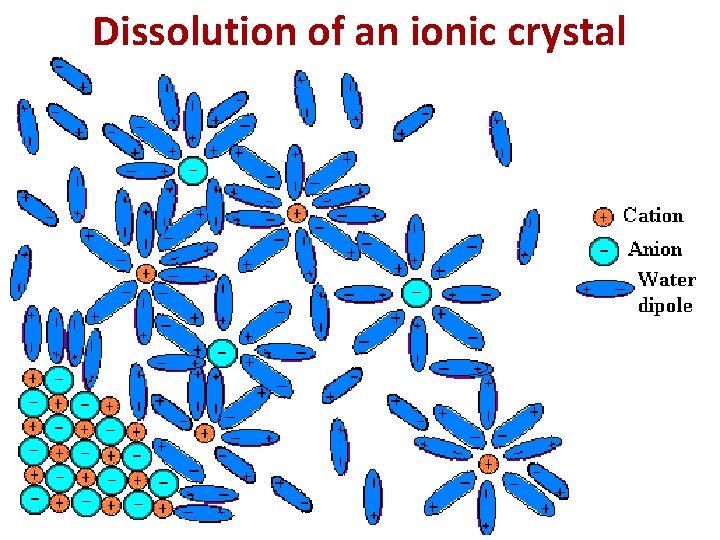
Dissolution of an ionic crystal
Dissociation of polar molecules
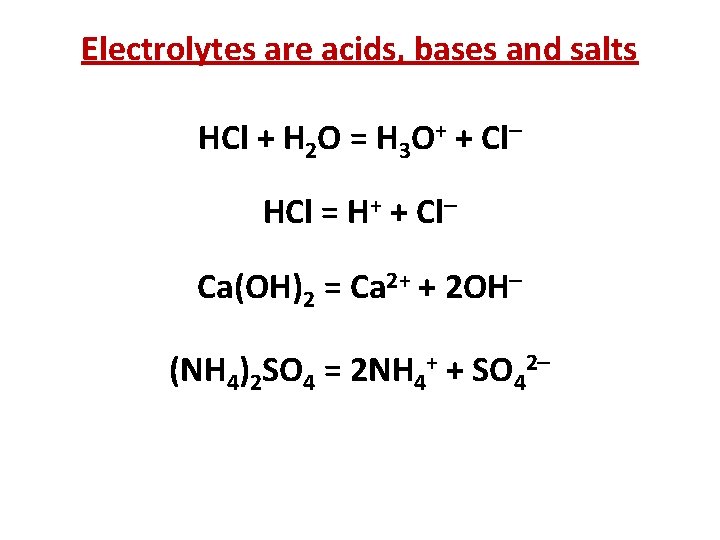
Electrolytes are acids, bases and salts HCl + H 2 O = H 3 O+ + Cl HCl = H+ + Cl Ca(OH)2 = Ca 2+ + 2 OH (NH 4)2 SO 4 = 2 NH 4+ + SO 42
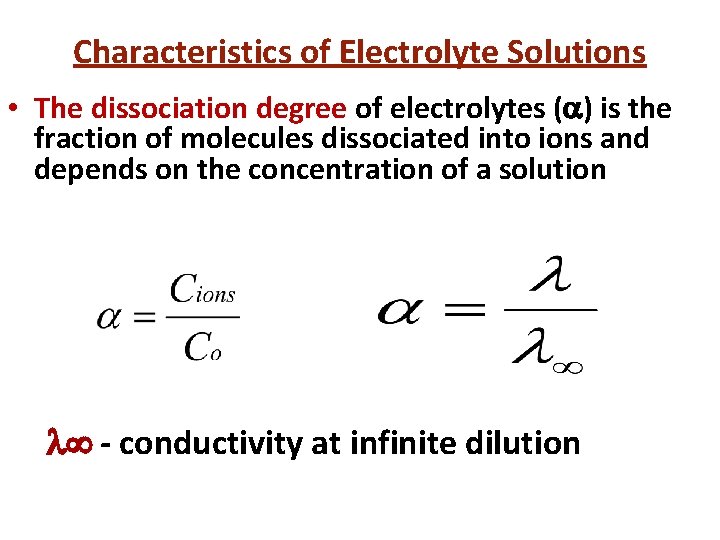
Characteristics of Electrolyte Solutions • The dissociation degree of electrolytes ( ) is the fraction of molecules dissociated into ions and depends on the concentration of a solution - conductivity at infinite dilution

Electrolytes are divided into: weak ( <0, 03) middle (0, 03< <0, 3) strong ( >0, 3)

Strong Electrolytes ØAlmost all the salts Ø Many inorganic acids (HCl, HBr, HI, H 2 SO 4, HNO 3, HCl. O 4 и др) ØHydroxides of alkali and alkaline-earth metals
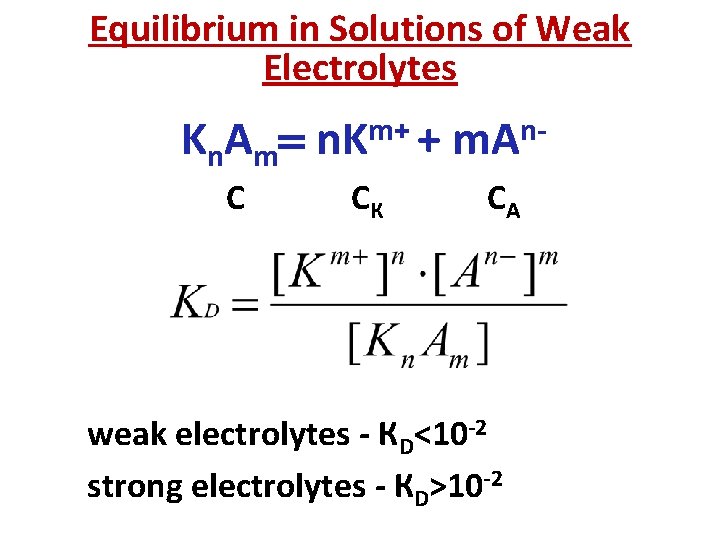
Equilibrium in Solutions of Weak Electrolytes K n A m С m+ n. K + СК nm. A СА weak electrolytes - КD<10 -2 strong electrolytes - КD>10 -2
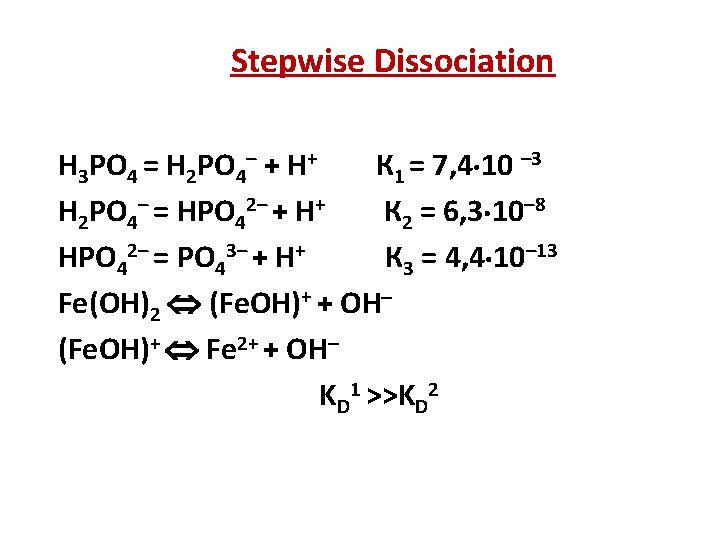
Stepwise Dissociation H 3 PO 4 = H 2 PO 4– + H+ К 1 = 7, 4 10 – 3 H 2 PO 4– = НPO 42– + H+ К 2 = 6, 3 10– 8 НPO 42– = PO 43– + H+ К 3 = 4, 4 10– 13 Fe(OH)2 (Fe. OH)+ + OH (Fe. OH)+ Fe 2+ + OH KD 1 >>KD 2

Soluble normal salt • Na 2 CO 3, Na. Cl, K 2 SO 4 and others are usual strong electrolytes and entirely dissociate in water • Acid salts dissociate into a metal cation and hidroanion: Na. HCO 3 = Na+ + HCO 3– • Basic salts dissociate into hydroxocation and anion: Mg. OHCl = Mg. OH+ + Cl–
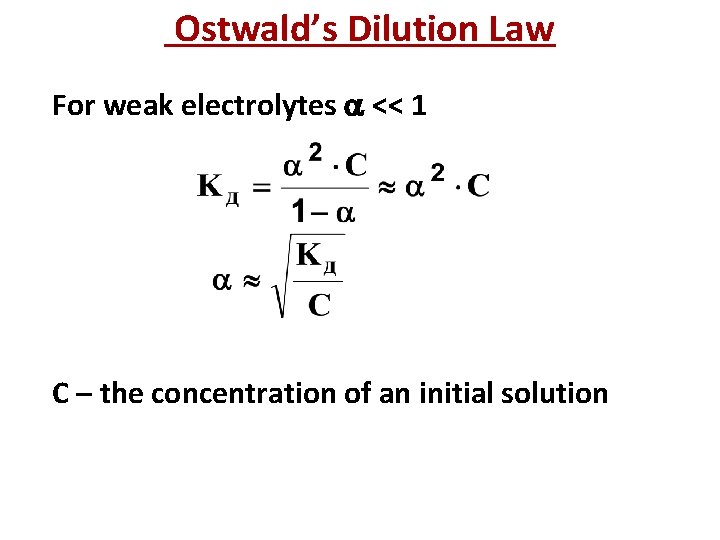
Ostwald’s Dilution Law For weak electrolytes << 1 С – the concentration of an initial solution
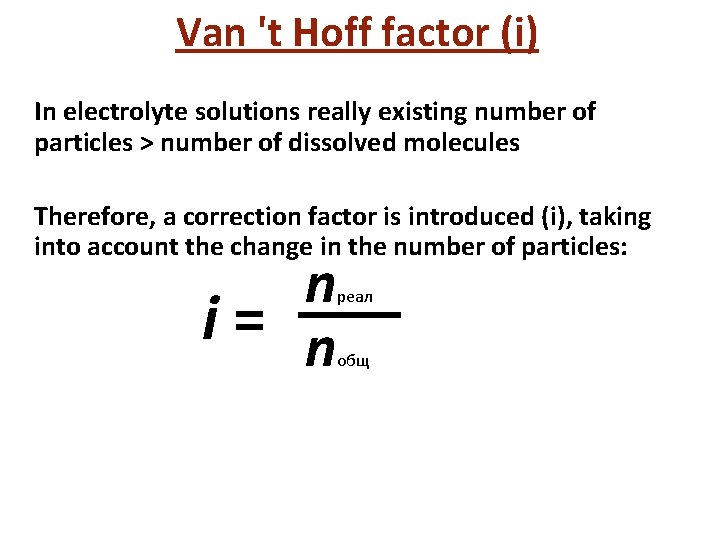
Van 't Hoff factor (i) In electrolyte solutions really existing number of particles > number of dissolved molecules Therefore, a correction factor is introduced (i), taking into account the change in the number of particles: n i= n реал общ
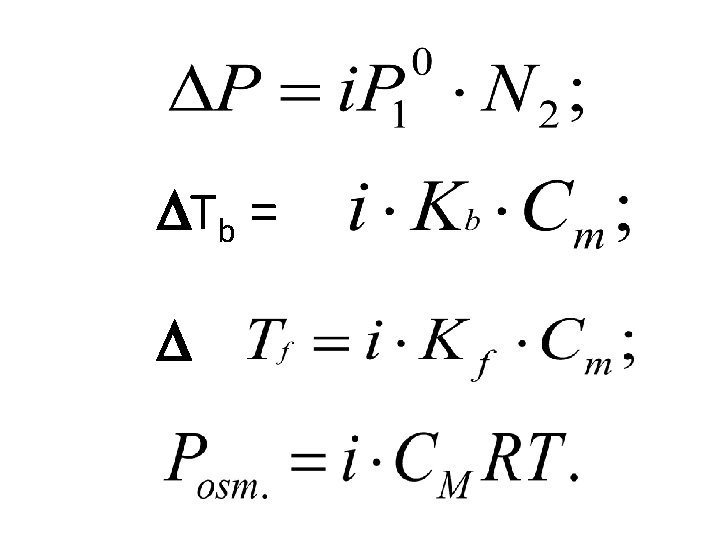
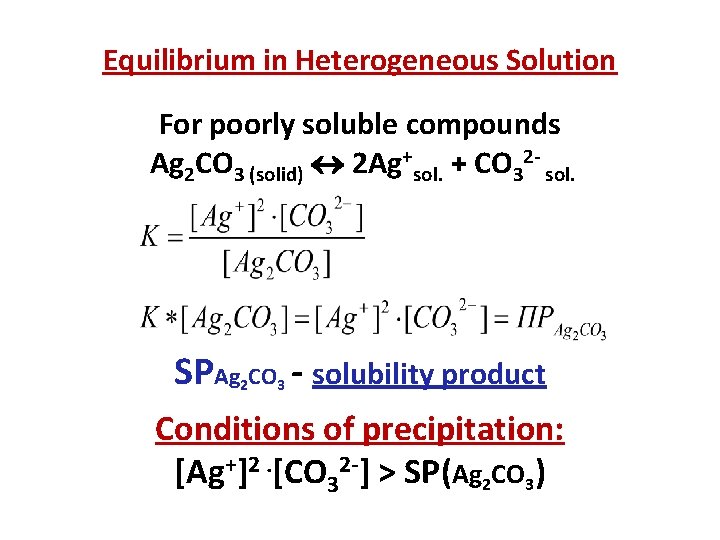
Equilibrium in Heterogeneous Solution For poorly soluble compounds Ag 2 СO 3 (solid) 2 Ag+sol. + CO 32 - sol. SPAg CO - solubility product 2 3 Conditions of precipitation: [Ag+]2. [CO 32 -] > SP(Ag 2 CO 3)
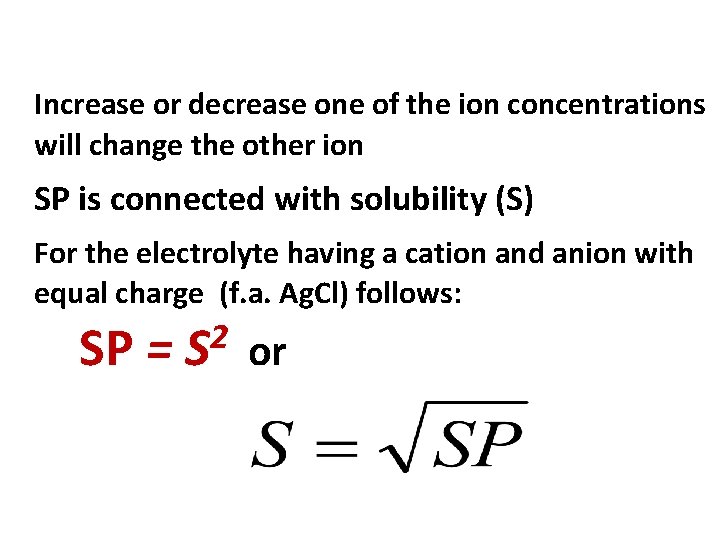
Increase or decrease one of the ion concentrations will change the other ion SP is connected with solubility (S) For the electrolyte having a cation and anion with equal charge (f. a. Ag. Cl) follows: SP = 2 S or
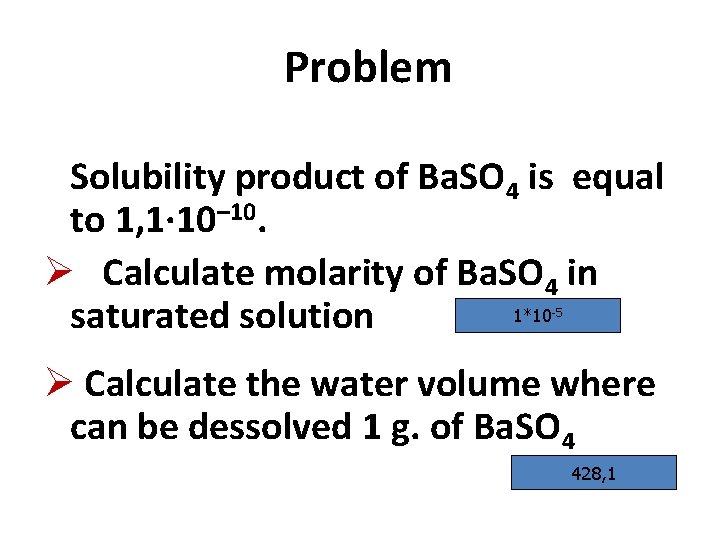
Problem Solubility product of Ba. SO 4 is equal to 1, 1∙ 10– 10. Ø Calculate molarity of Ba. SO 4 in 1*10 saturated solution -5 Ø Calculate the water volume where can be dessolved 1 g. of Ba. SO 4 428, 1

Exchange reactions in electrolyte solutions
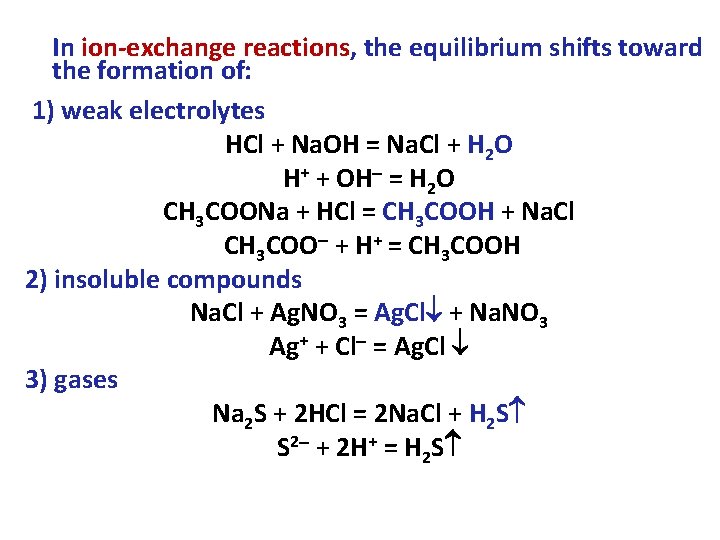
In ion-exchange reactions, the equilibrium shifts toward the formation of: 1) weak electrolytes HCl + Na. OH = Na. Cl + H 2 O H+ + OH = H 2 O CH 3 COONa + HCl = CH 3 COOH + Na. Cl CH 3 COO + H+ = CH 3 COOH 2) insoluble compounds Na. Cl + Ag. NO 3 = Ag. Cl + Na. NO 3 Ag+ + Cl = Ag. Cl 3) gases Na 2 S + 2 HCl = 2 Na. Cl + H 2 S S 2 + 2 H+ = H 2 S
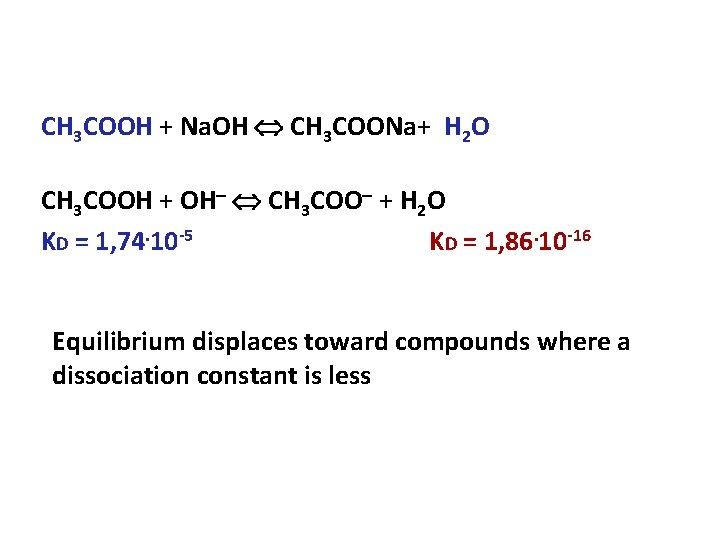
CH 3 COOH + Na. OH CH 3 COONa+ H 2 O CH 3 COOH + OH CH 3 COO + H 2 O KD = 1, 74. 10 -5 KD = 1, 86. 10 -16 Equilibrium displaces toward compounds where a dissociation constant is less
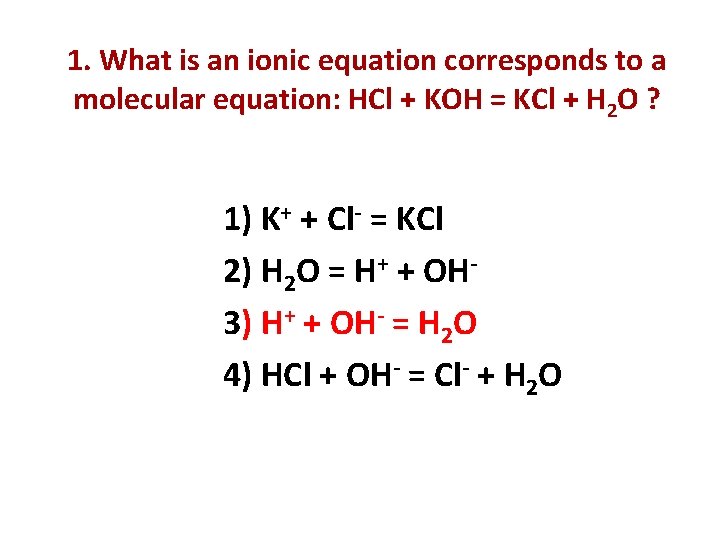
1. What is an ionic equation corresponds to a molecular equation: HCl + KOH = KCl + H 2 O ? 1) K+ + Cl- = KCl 2) H 2 O = H+ + OH 3) H+ + OH- = H 2 O 4) HCl + OH- = Cl- + H 2 O
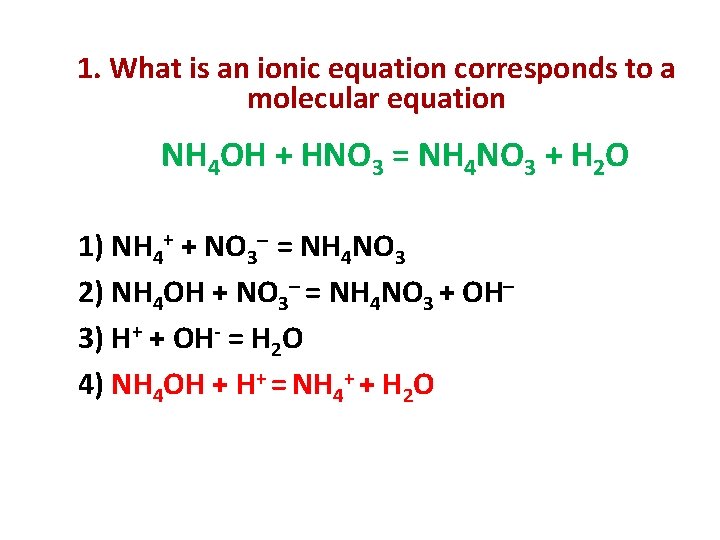
1. What is an ionic equation corresponds to a molecular equation NH 4 OH + HNO 3 = NH 4 NO 3 + H 2 O 1) NH 4+ + NO 3 = NH 4 NO 3 2) NH 4 OH + NO 3 = NH 4 NO 3 + OH 3) H+ + OH- = H 2 O 4) NH 4 OH + H+ = NH 4+ + H 2 O

Hydrolysis of salts
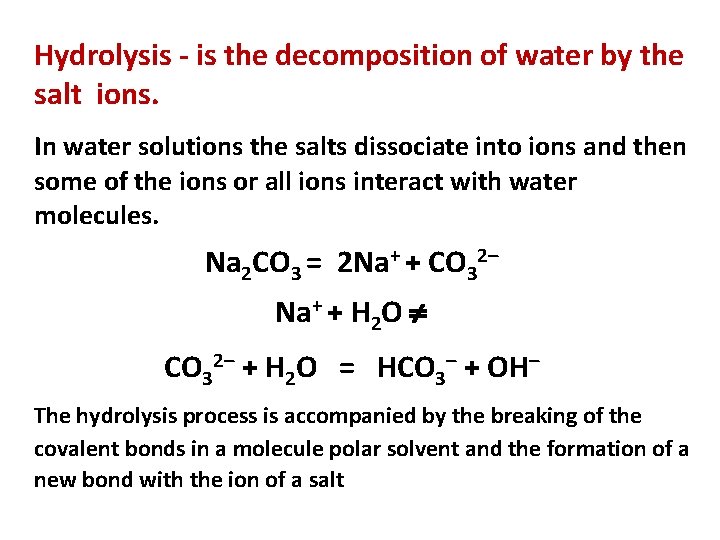
Hydrolysis - is the decomposition of water by the salt ions. In water solutions the salts dissociate into ions and then some of the ions or all ions interact with water molecules. Na 2 CO 3 = 2 Na+ + CO 32– Na+ + H 2 O CO 32– + H 2 O = HCO 3– + OH– The hydrolysis process is accompanied by the breaking of the covalent bonds in a molecule polar solvent and the formation of a new bond with the ion of a salt

Strong hydrolysis is observed: Ø for the cations which can form weak bases such as Al 3+, Fe 3+, Bi 3+ and others. Ø for the anions which can form weak acids such CO 32–, SO 32–, NO 2–, CN–, S 2– and others. Al 3+ + H 2 O = Al(OH)2+ + H+ SO 32– + H 2 O = HSO 32– + OH– Ø not hydrolyzed : — the cations of strong bases such as Na+, Ca 2+ and others — the anions of strong acids such as (Cl– , Br– , I– NO 3–, SO 42–, Mn. O 4–, Cl. O 4–, Cr 2 O 72–).
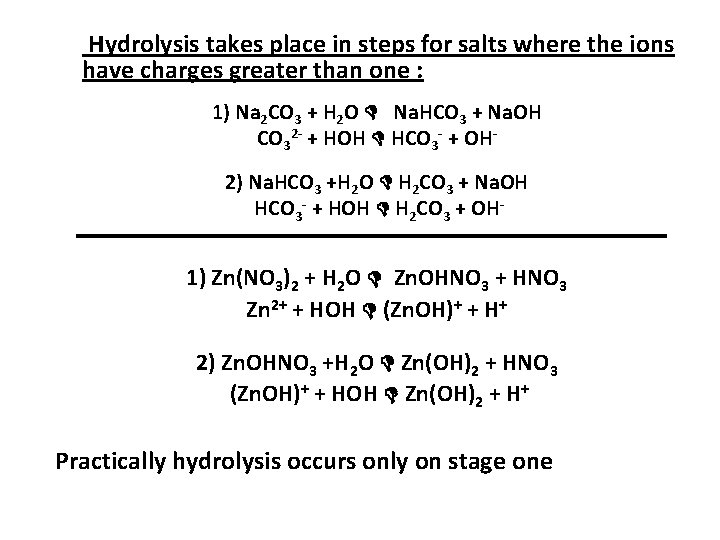
Hydrolysis takes place in steps for salts where the ions have charges greater than one : 1) Na 2 CO 3 + H 2 O Na. HCO 3 + Na. OH CO 32 - + HOH HCO 3 - + OH 2) Na. HCO 3 +H 2 O H 2 CO 3 + Na. OH HCO 3 - + HOH H 2 CO 3 + OH- 1) Zn(NO 3)2 + H 2 O Zn. OHNO 3 + HNO 3 Zn 2+ + HOH (Zn. OH)+ + H+ 2) Zn. OHNO 3 +H 2 O Zn(OH)2 + HNO 3 (Zn. OH)+ + HOH Zn(OH)2 + H+ Practically hydrolysis occurs only on stage one
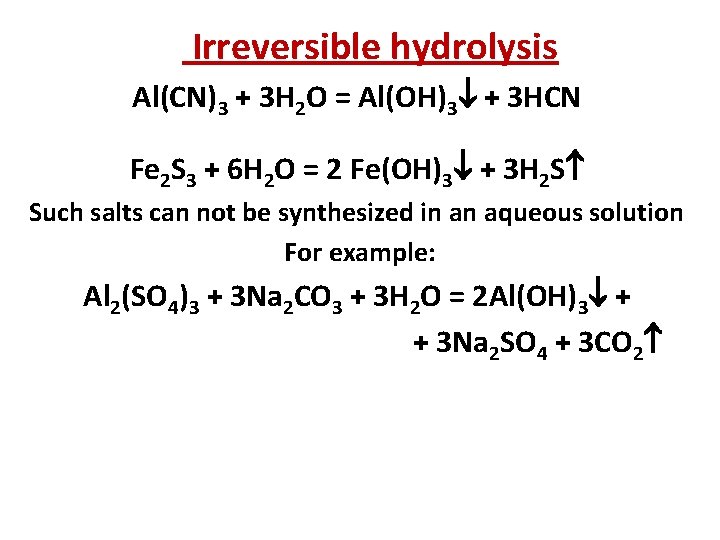
Irreversible hydrolysis Al(CN)3 + 3 Н 2 О = Al(OH)3 + 3 HCN Fe 2 S 3 + 6 H 2 O = 2 Fe(OH)3 + 3 H 2 S Such salts can not be synthesized in an aqueous solution For example: Al 2(SO 4)3 + 3 Na 2 CO 3 + 3 Н 2 О = 2 Al(OH)3 + + 3 Na 2 SO 4 + 3 CО 2

Factors affecting the hydrolysis Hydrolysis can be enhanced if: 1) to raise the temperature 2) to dilute the solution with water 3) to add a substance interacting with the hydrolysis products
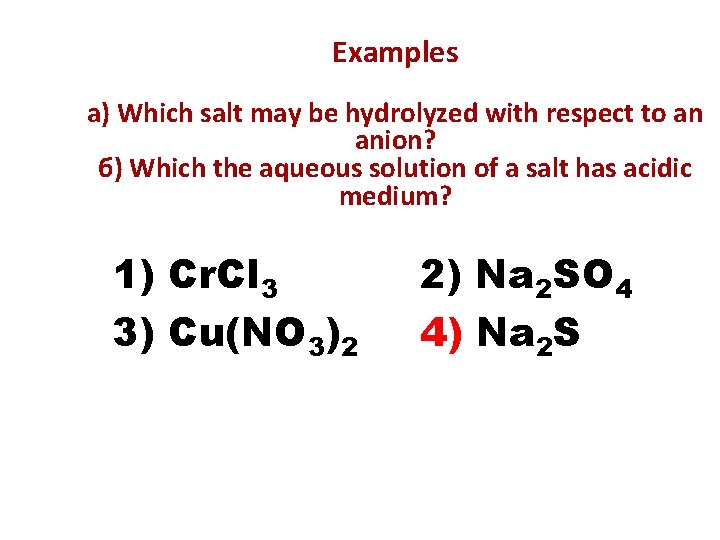
Examples а) Which salt may be hydrolyzed with respect to an anion? б) Which the aqueous solution of a salt has acidic medium? 1) Cr. Cl 3 3) Cu(NO 3)2 2) Nа 2 SO 4 4) Na 2 S
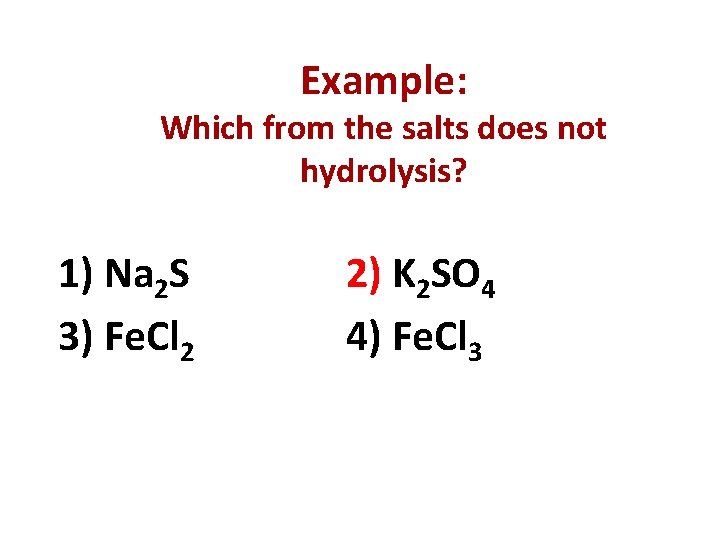
Example: Which from the salts does not hydrolysis? 1) Na 2 S 3) Fe. Cl 2 2) K 2 SO 4 4) Fe. Cl 3
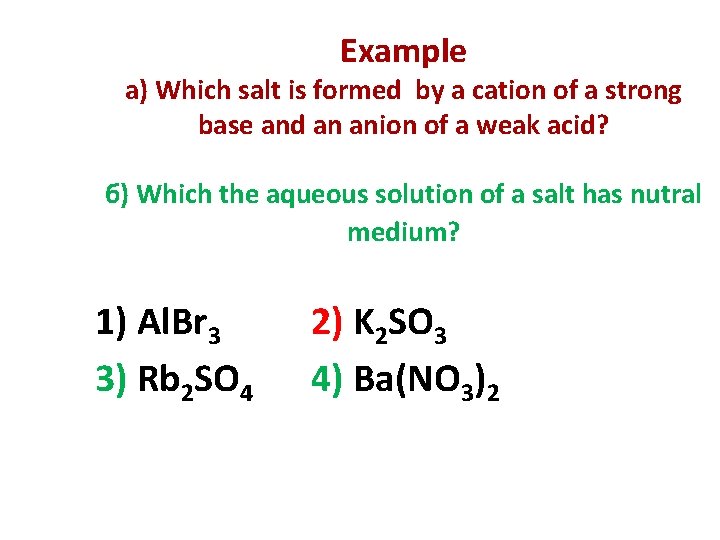
Example а) Which salt is formed by a cation of a strong base and an anion of a weak acid? б) Which the aqueous solution of a salt has nutral medium? 1) Al. Br 3 3) Rb 2 SO 4 2) K 2 SO 3 4) Ba(NO 3)2
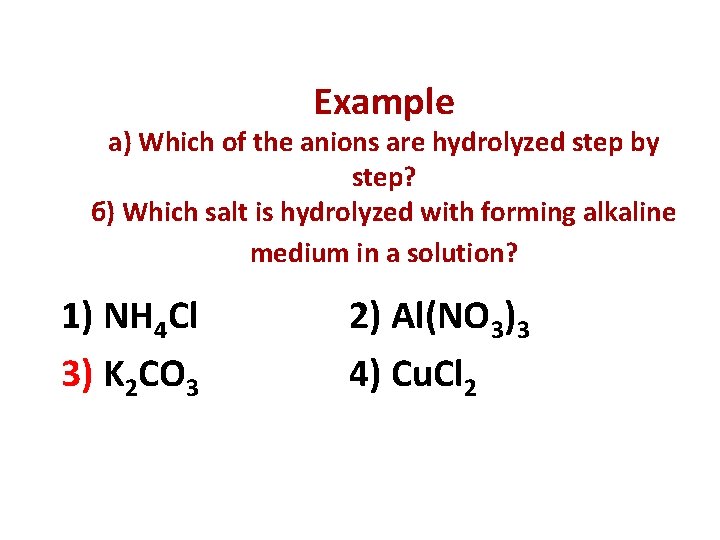
Example а) Which of the anions are hydrolyzed step by step? б) Which salt is hydrolyzed with forming alkaline medium in a solution? 1) NH 4 Cl 3) K 2 CO 3 2) Al(NO 3)3 4) Cu. Cl 2

Example What a salt is hydrolyzed completely? 1) K 2 Cr. O 4 2) Al. F 3 3) Al(NO 3)3 4) Al 2(SO 4)3
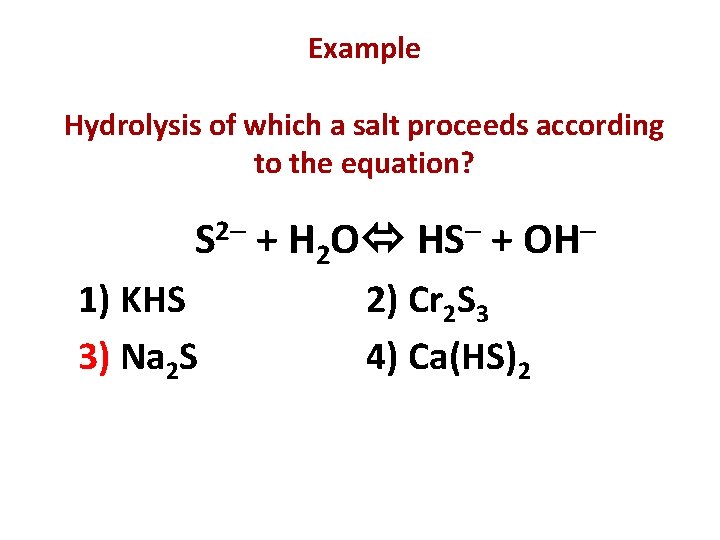
Example Hydrolysis of which a salt proceeds according to the equation? 2 S 1) KHS 3) Na 2 S + H 2 O HS + OH 2) Cr 2 S 3 4) Ca(HS)2
- Slides: 64