SOLUTIONS What is a solution A homogenous mixture






- Slides: 15

SOLUTIONS

What is a solution? • A homogenous mixture: • Composed of at least two different substances that can be separated using a physical technique. • The substances cannot be distinguished. • Homogenous mixtures are composed of a: • Solute, (substance which is dissolved). • Solvent, (liquid which dissolves the solute). • When you mix a solute and a solvent, two things can happen: 1. The solute dissolves and separates in the solvent. 2. The solute does not dissolve and forms a precipitate.

Common Solutions • Solution can exist in any state of matter. • Tap water • Water is the solvent, salt is the solute. • Air • Nitrogen is the solvent, oxygen, water vapour, carbon dioxide are solutes. • Brass • Copper is the solvent, zinc is the solute. • Aqueous solutions are those in which the solvent is water.

Key Things to Remember Solvent → Liquid doing the dissolving Solute → Substance being dissolved Solution → Combination of the solvent and the solute together. Example: Water and koolaid. Water is solvent, koolaid is solute, the solution is your drink.

Precipitates • Precipitates can sometimes form within a solution. • Definition: formation of a solid in a solution. • Can form due to many things: (Key ones you need to know ) • Solute you are trying to dissolve in solvent is insoluble (can’t dissolve). • Sand, oil, etc. are insoluble. • Too much of a soluble solute is introduced to a solvent and the solution becomes supersaturated. • Definition: solution that has more of a dissolved solute that can de dissolved by the solvent.

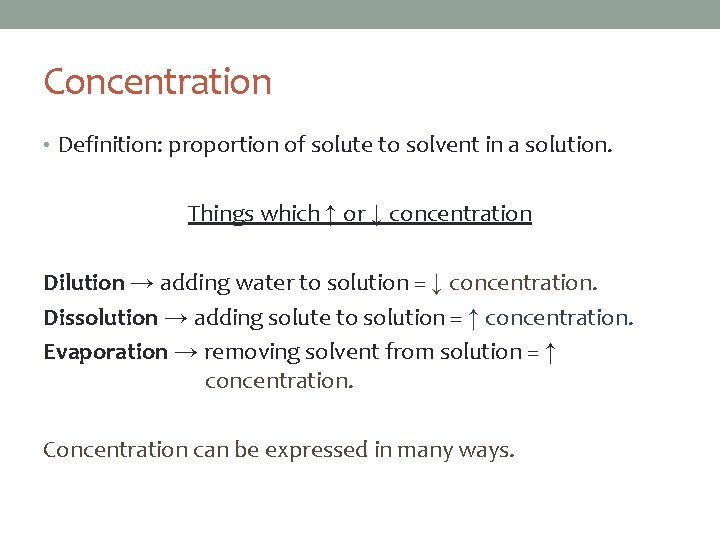
Concentration • Definition: proportion of solute to solvent in a solution. Things which ↑ or ↓ concentration Dilution → adding water to solution = ↓ concentration. Dissolution → adding solute to solution = ↑ concentration. Evaporation → removing solvent from solution = ↑ concentration. Concentration can be expressed in many ways.

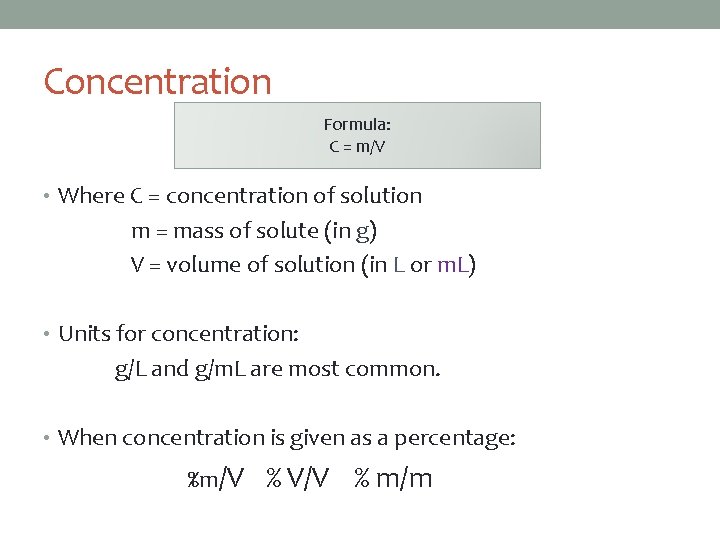
Concentration Formula: C = m/V • Where C = concentration of solution m = mass of solute (in g) V = volume of solution (in L or m. L) • Units for concentration: g/L and g/m. L are most common. • When concentration is given as a percentage: %m/V % V/V % m/m

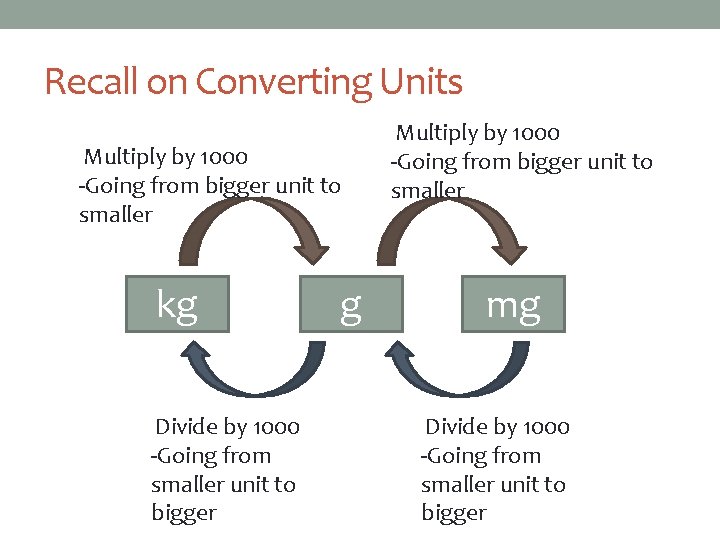
Recall on Converting Units Multiply by 1000 -Going from bigger unit to smaller kg Divide by 1000 -Going from smaller unit to bigger g Multiply by 1000 -Going from bigger unit to smaller mg Divide by 1000 -Going from smaller unit to bigger

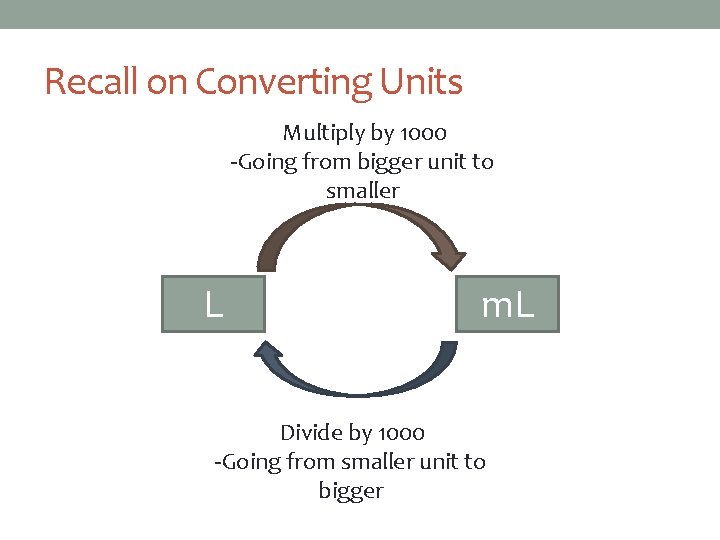
Recall on Converting Units Multiply by 1000 -Going from bigger unit to smaller L m. L Divide by 1000 -Going from smaller unit to bigger

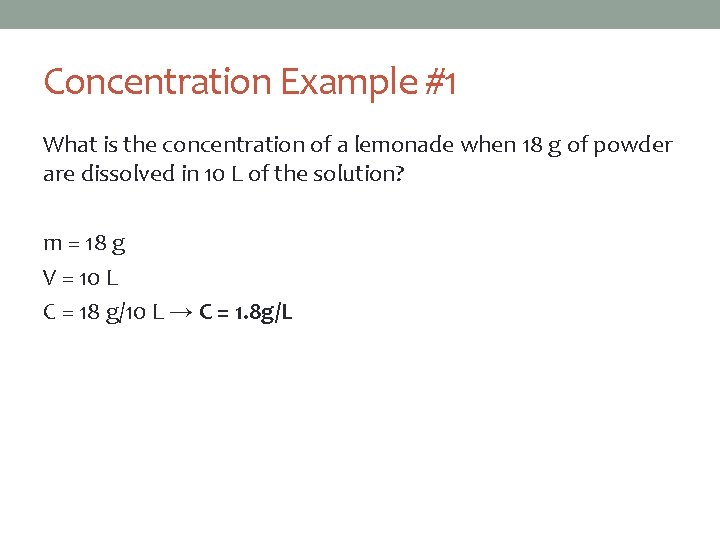
Concentration Example #1 What is the concentration of a lemonade when 18 g of powder are dissolved in 10 L of the solution? m = 18 g V = 10 L C = 18 g/10 L → C = 1. 8 g/L

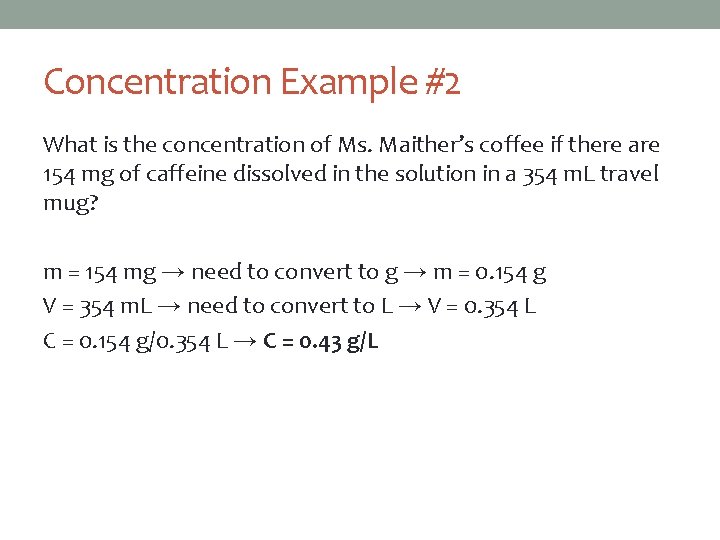
Concentration Example #2 What is the concentration of Ms. Maither’s coffee if there are 154 mg of caffeine dissolved in the solution in a 354 m. L travel mug? m = 154 mg → need to convert to g → m = 0. 154 g V = 354 m. L → need to convert to L → V = 0. 354 L C = 0. 154 g/0. 354 L → C = 0. 43 g/L

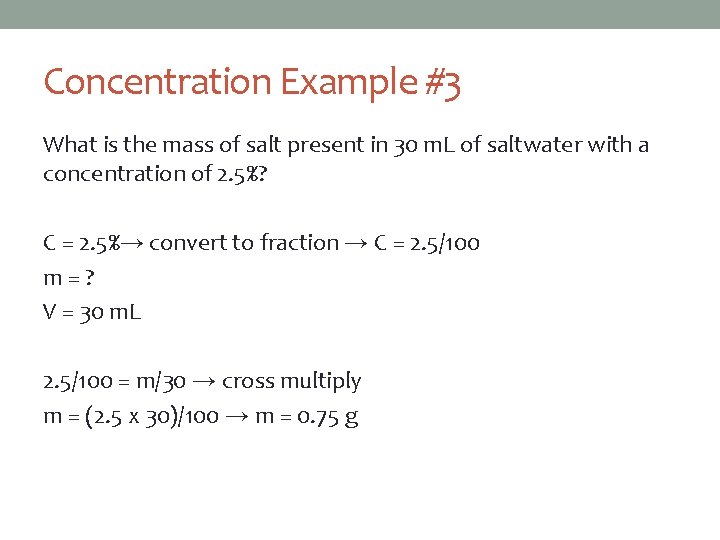
Concentration Example #3 What is the mass of salt present in 30 m. L of saltwater with a concentration of 2. 5%? C = 2. 5%→ convert to fraction → C = 2. 5/100 m=? V = 30 m. L 2. 5/100 = m/30 → cross multiply m = (2. 5 x 30)/100 → m = 0. 75 g

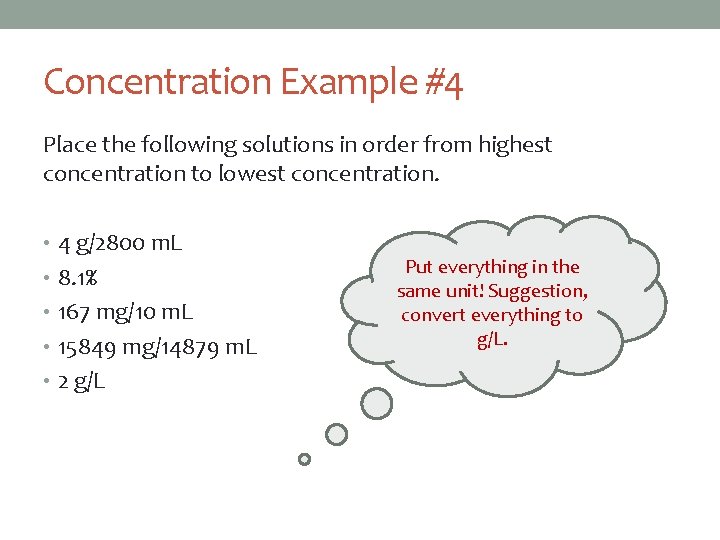
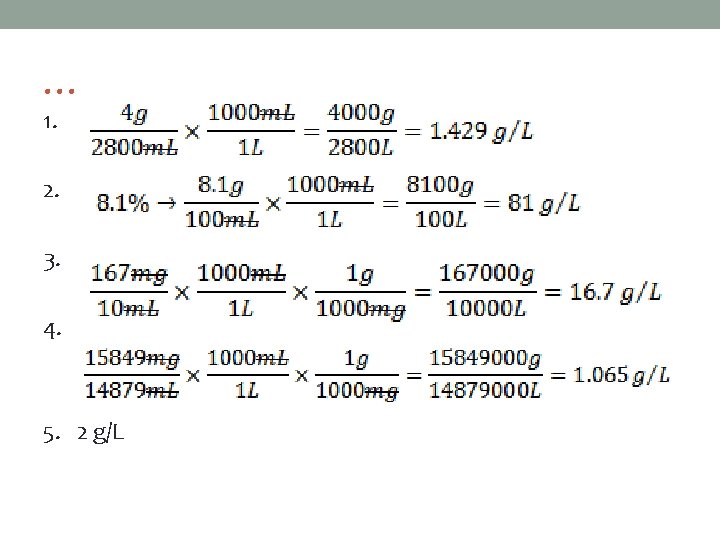
Concentration Example #4 Place the following solutions in order from highest concentration to lowest concentration. • 4 g/2800 m. L • 8. 1% • 167 mg/10 m. L • 15849 mg/14879 m. L • 2 g/L Put everything in the same unit! Suggestion, convert everything to g/L.

… 1. 2. 3. 4. 5. 2 g/L

Parts Per Million (ppm) • Unit of concentration used when the solute is much, much smaller than the amount of the solution. (When the solution is solid) • In an aqueous solution, 1 ppm is equal to about 1 mg of solute in 1 L of solution. 1 ppm = 1 g/1000 L or 1 mg/L