Solutions Water is good solvent How soap works













- Slides: 21

Solutions *Water is good solvent *How soap works *Colloids *Dilute and concentrated solutions *Saturated , unsaturated and supersaturated solutions

Solute – The substance that is dissolved Solvent – The substance that dissolves Solute + Solvent = SOLUTION Salt , Solute Water , Solvent

“like dissolves like. ” Polar solutes tend to dissolve in polar solvents Non-polar solutes tend to dissolve in non-polar solvents

Water is a good solvent ** Water is polar it dissolves polar substances. Explanation : positive end of water is attracted to negative end of polar solute and negative end of water is attracted to positive end of solute

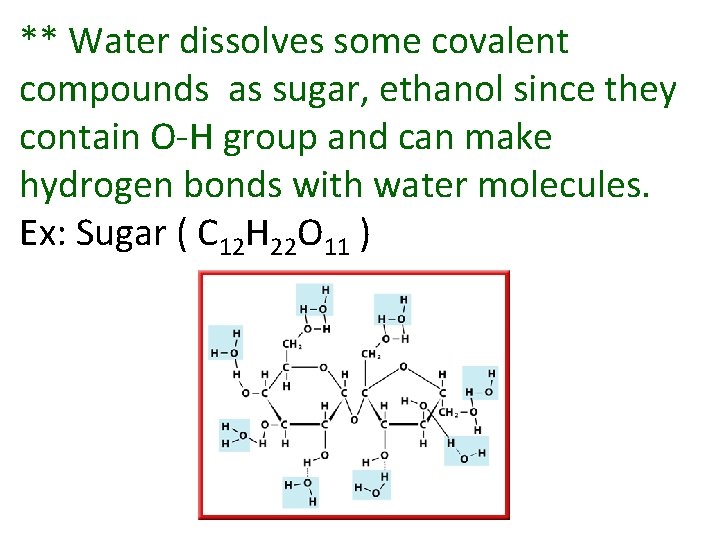
** Water dissolves some covalent compounds as sugar, ethanol since they contain O-H group and can make hydrogen bonds with water molecules. Ex: Sugar ( C 12 H 22 O 11 )

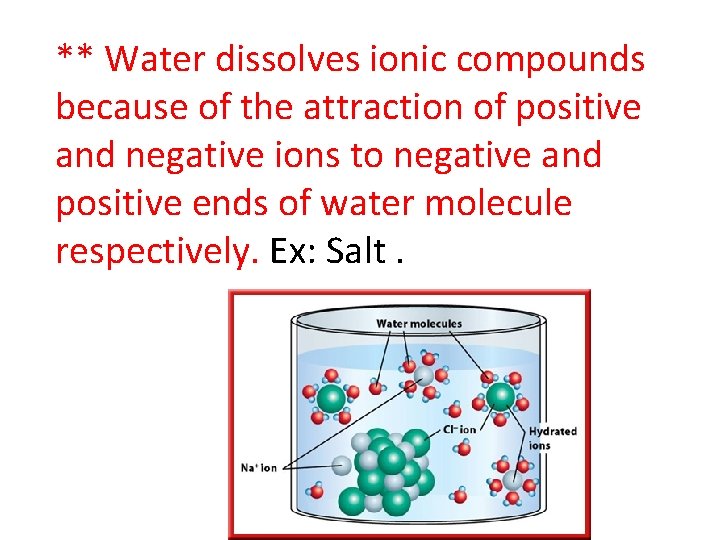
** Water dissolves ionic compounds because of the attraction of positive and negative ions to negative and positive ends of water molecule respectively. Ex: Salt.

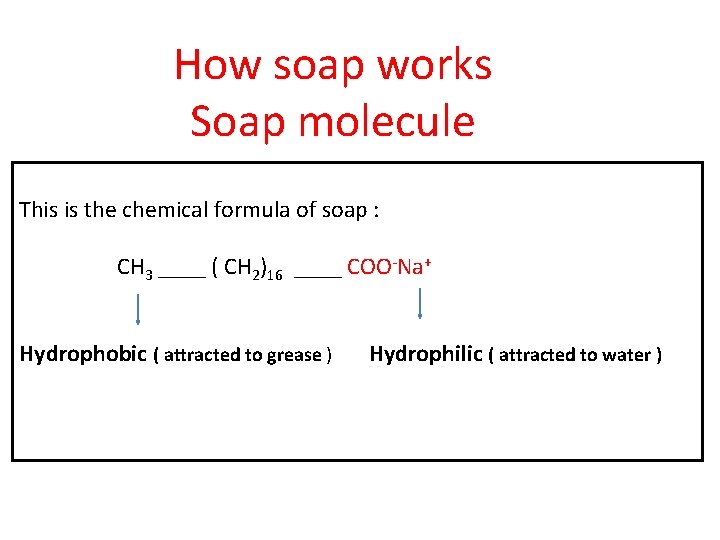
How soap works Soap molecule This is the chemical formula of soap : CH 3 ____ ( CH 2)16 ____ COO-Na+ Hydrophobic ( attracted to grease ) Hydrophilic ( attracted to water )

The polar ( charged) end of soap ( hydrophilic) is attracted to water molecules. The non-polar end ( hydrophobic) is attracted to grease. By scrubbing and rubbing soap molecules will carry away grease with water molecules.

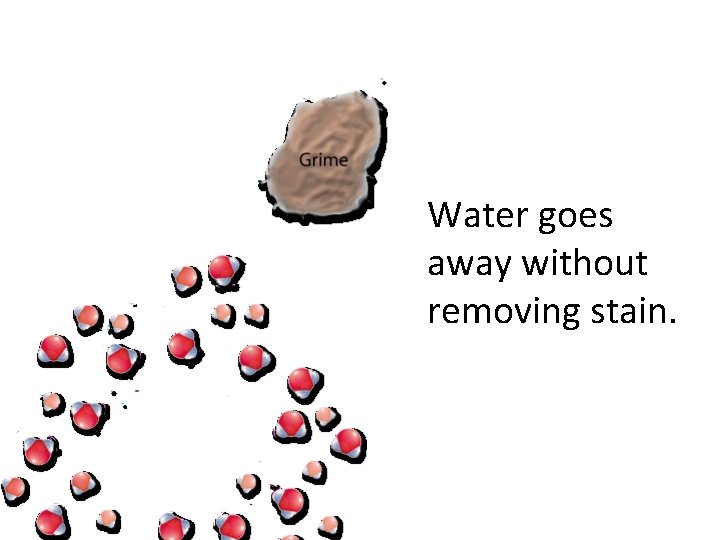
Dirt , grease or stains that are insoluble in water.

Water goes away without removing stain.

Soap soluble, the non-polar end of soap is attracted to grime.

The charged end ( polar ) of the soap molecule is attracted to water.

Solution, colloid and suspension A solution contains very small particles of the solute and light can pass through it. Ex: salt or sugar in water.

A colloid contains particles of intermediate size that will not settle down as in suspension and it scatters light ( Tyndall effect) Ex: milk

A suspension contains particles of large size which will settle down. Ex: sand or chalk and water.

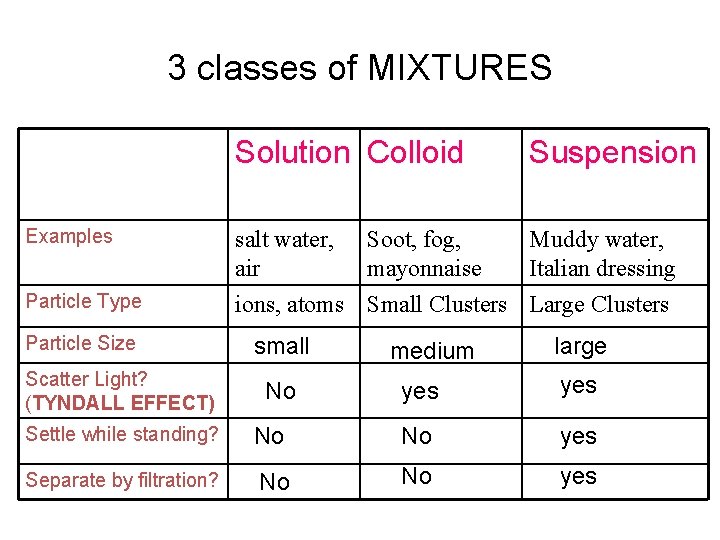
3 classes of MIXTURES Solution Colloid Examples Particle Type Particle Size Scatter Light? (TYNDALL EFFECT) Suspension salt water, Soot, fog, Muddy water, air mayonnaise Italian dressing ions, atoms Small Clusters Large Clusters small No medium large yes Settle while standing? No No yes Separate by filtration? No No yes

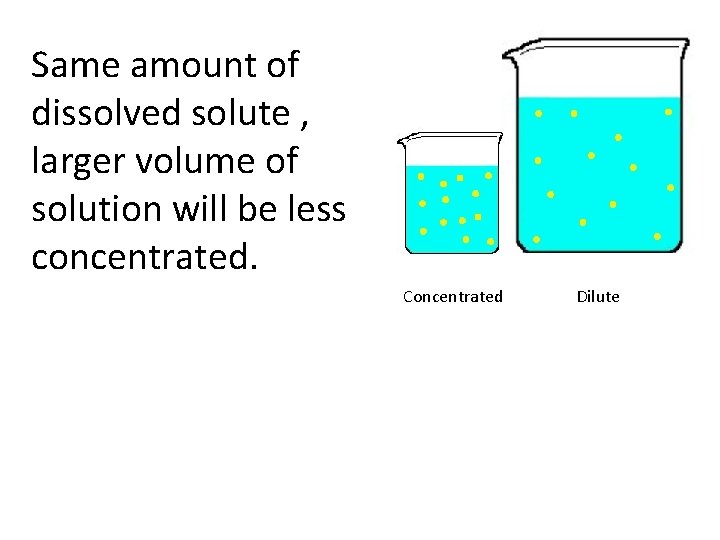
Concentration of a solution Concentration is related to amount of solute dissolved and volume of solution.

A dilute solution is that which contains small number of dissolved particles per unit volume of solution. A concentrated solution contains large number of dissolved particles per unit volume of solution.

Same amount of dissolved solute , larger volume of solution will be less concentrated. Concentrated Dilute

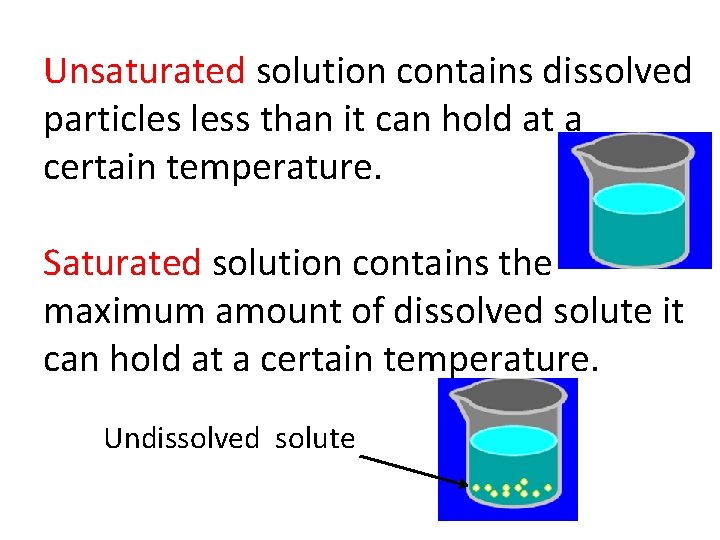
Unsaturated solution contains dissolved particles less than it can hold at a certain temperature. Saturated solution contains the maximum amount of dissolved solute it can hold at a certain temperature. Undissolved solute

Supersaturated solution contains more dissolved particles that it is capable to hold at a certain temperature. ( It is unstable if shaken all extra dissolved particles will settle down)