Solutions Suspensions Colloids Oh My Mixtures Pure Substances

Solutions & Suspensions & Colloids - Oh My! Mixtures
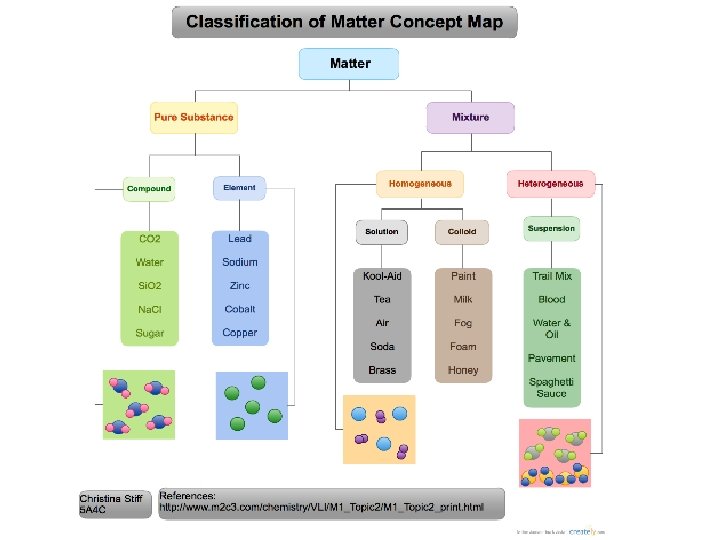
Pure Substances Elements One kind of atom Could be diatomic Compounds 2 or more types of atoms In a set ratio

Mixtures: variable composition Most things in nature Substances held together by physical forces Can be separated by physical means Types: Heterogeneous ▪ Visibly different substances or phases / states ▪ Trail mix, salad Homogeneous ▪ Uniform appearance and composition ▪ Kool-aid, sugar water

Mixtures: Solutions Solution A type of homogeneous mixture One substance (solute) is dissolved in another (solvent) ▪ Ex. ▪ Solid in liquid: tea, coffee ▪ Solid in solid: brass, bronze ▪ Alloy – metal in metal ▪ Amalgams: special type of alloy; mercury + another metal

Types of Solutions Solute Liquid Solid Gas Solid Solvent Liquid Gas Gas Solid Example Vinegar (acetic acid in water) Salt water Soft drinks (CO 2 in water) Water in air Smog Air (oxygen, nitrogen, etc) Bronze (alloy)
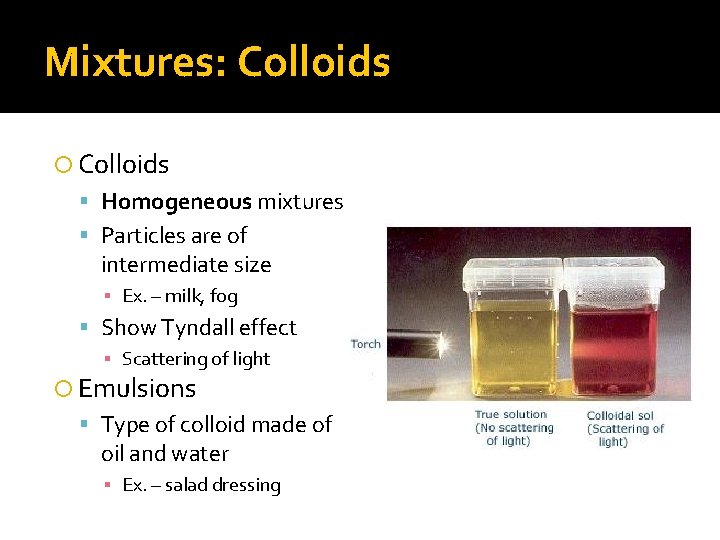
Mixtures: Colloids Homogeneous mixtures Particles are of intermediate size ▪ Ex. – milk, fog Show Tyndall effect ▪ Scattering of light Emulsions Type of colloid made of oil and water ▪ Ex. – salad dressing

Mixtures: Suspensions Heterogeneous mixtures Particles are large enough to settle out ▪ Sedimentation ▪ Ex. sand in water – solid in liquid aerosols – liquid in gas
Concept Map Create a concept map of matter Include examples Matter
Concept Map Create a concept map of matter Include examples Matter Pure Mixture
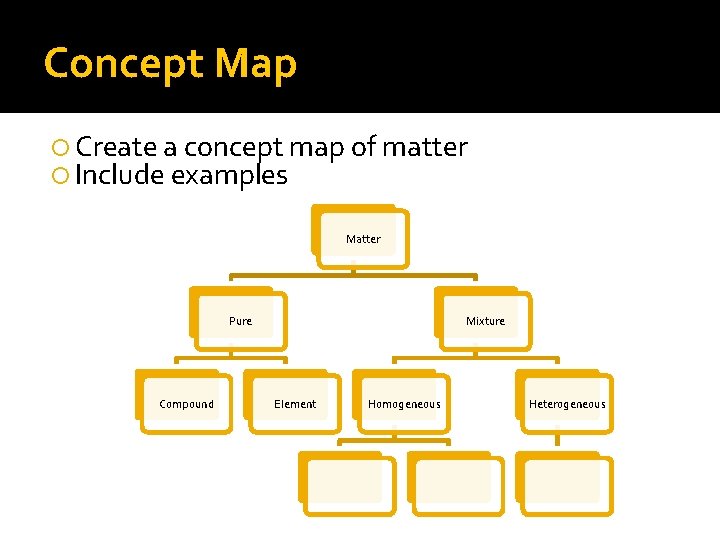
Concept Map Create a concept map of matter Include examples Matter Pure Compound Mixture Element Homogeneous Heterogeneous
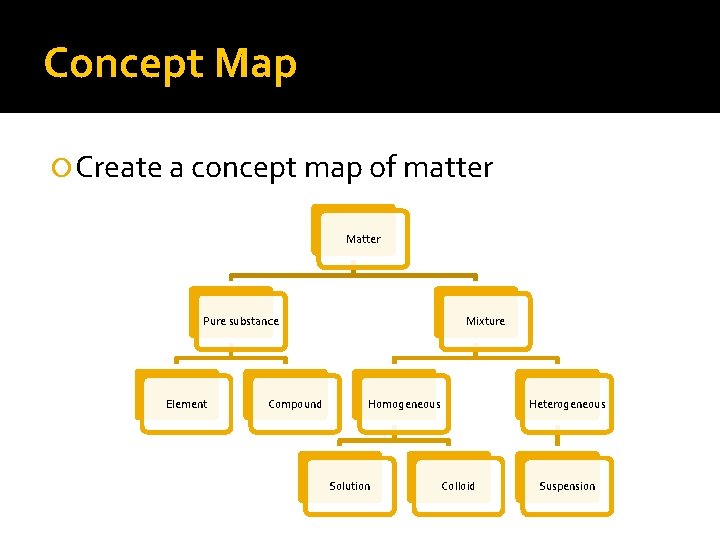
Concept Map Create a concept map of matter Matter Pure substance Element Compound Mixture Homogeneous Solution Heterogeneous Colloid Suspension
- Slides: 12