Solutions Solutions Homogeneous mixtures of 2 or more















- Slides: 32

Solutions

Solutions Homogeneous mixtures of 2 or more substances Can be: Solid Sterling Silver Gas Liquid ost m d e s U Air ** y in l t n e u q e fr y** r t s i m e h c

Solution Components: Solvent the component present in the greatest amount Solute all other components dissolved in solvent

Aqueous Solutions Solvent Solute WATER Whatever is dissolved in the water In chemical equations, denoted as aqueous (aq)

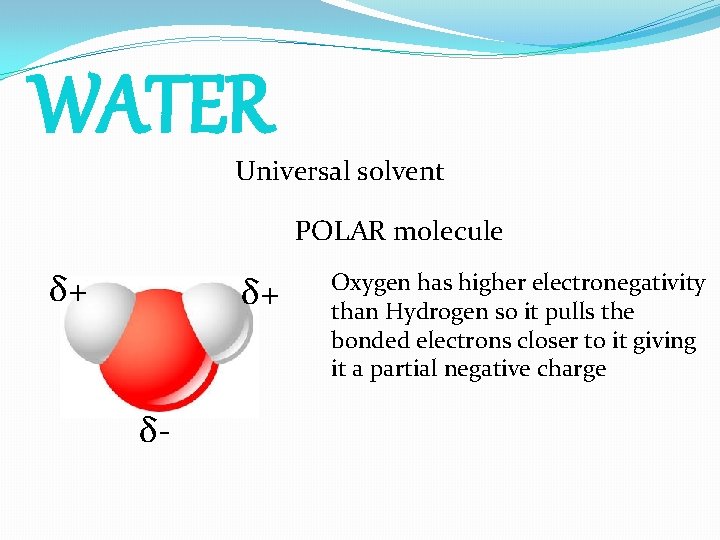
WATER Universal solvent POLAR molecule δ+ δ+ δ- Oxygen has higher electronegativity than Hydrogen so it pulls the bonded electrons closer to it giving it a partial negative charge

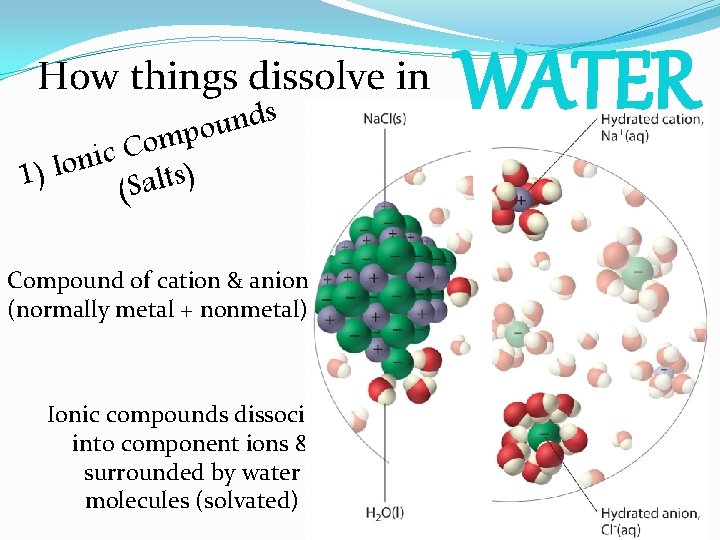
How things dissolve in s d n u o p m o C c i n o I ) ) s 1 t l a (S Compound of cation & anion (normally metal + nonmetal) Ionic compounds dissociate into component ions & surrounded by water molecules (solvated) WATER

How things dissolve in s d n u o p m o C t n e l a v o ) s C e ) l 2 u c e l o M ( WATER Compounds of nonmetals Molecular substances dissolve without forming ions water molecules surround molecules *EXCEPTION: Acids/Bases

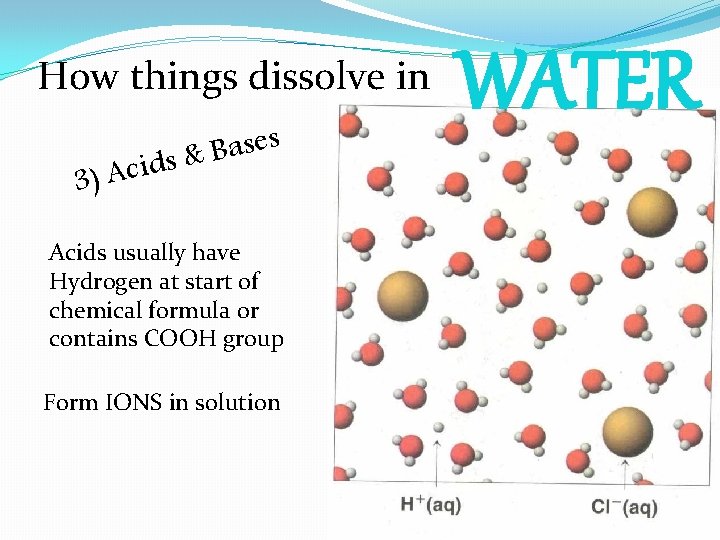
How things dissolve in s e s a B ids & 3) Ac Acids usually have Hydrogen at start of chemical formula or contains COOH group Form IONS in solution WATER

STRONG ACID/BASES Completely dissociate WEAK ACID/BASES Partially dissociate

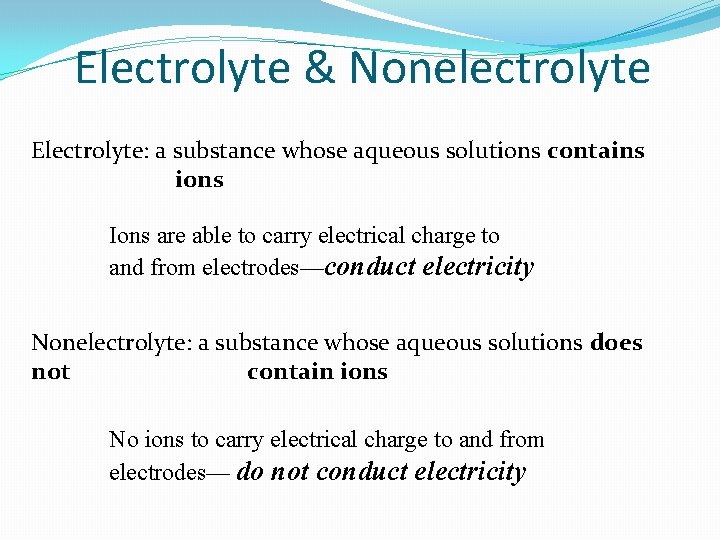
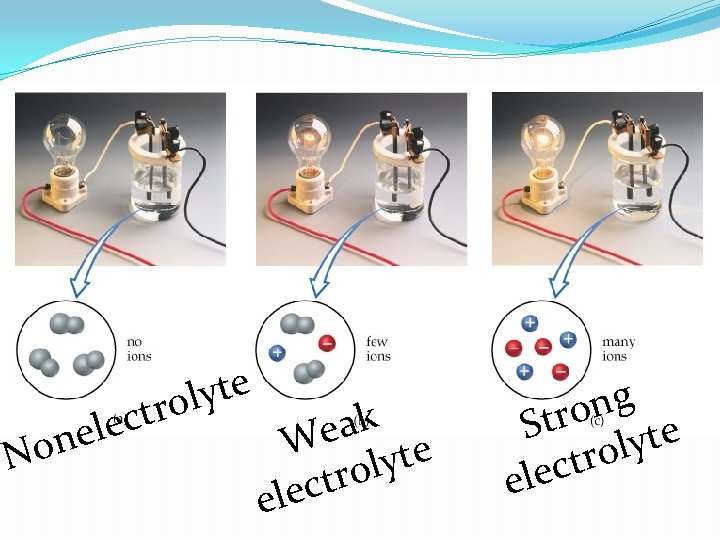
Electrolyte & Nonelectrolyte Electrolyte: a substance whose aqueous solutions contains ions Ions are able to carry electrical charge to and from electrodes—conduct electricity Nonelectrolyte: a substance whose aqueous solutions does not contain ions No ions to carry electrical charge to and from electrodes— do not conduct electricity

N c e l e n o e t y l tro k a e W e t y l o r t c ele g n Stro lyte o r t elec

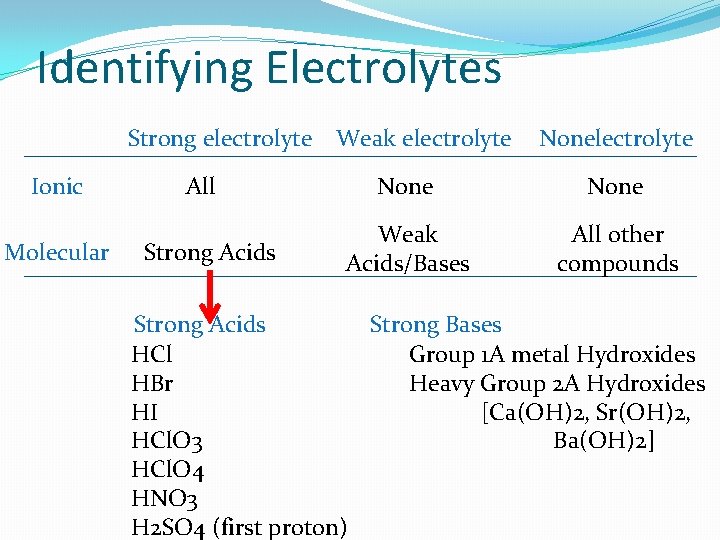
Identifying Electrolytes Strong electrolyte Weak electrolyte Ionic Molecular All Strong Acids Nonelectrolyte None Weak Acids/Bases All other compounds Strong Acids Strong Bases HCl Group 1 A metal Hydroxides HBr Heavy Group 2 A Hydroxides HI [Ca(OH)2, Sr(OH)2, HCl. O 3 Ba(OH)2] HCl. O 4 HNO 3 H 2 SO 4 (first proton)

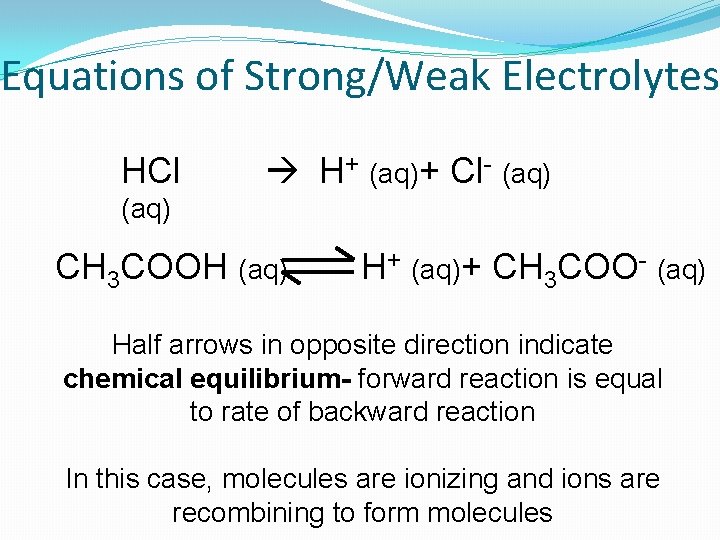
Equations of Strong/Weak Electrolytes HCl H+ (aq)+ Cl- (aq) CH 3 COOH (aq) H+ (aq)+ CH 3 COO- (aq) Half arrows in opposite direction indicate chemical equilibrium- forward reaction is equal to rate of backward reaction In this case, molecules are ionizing and ions are recombining to form molecules

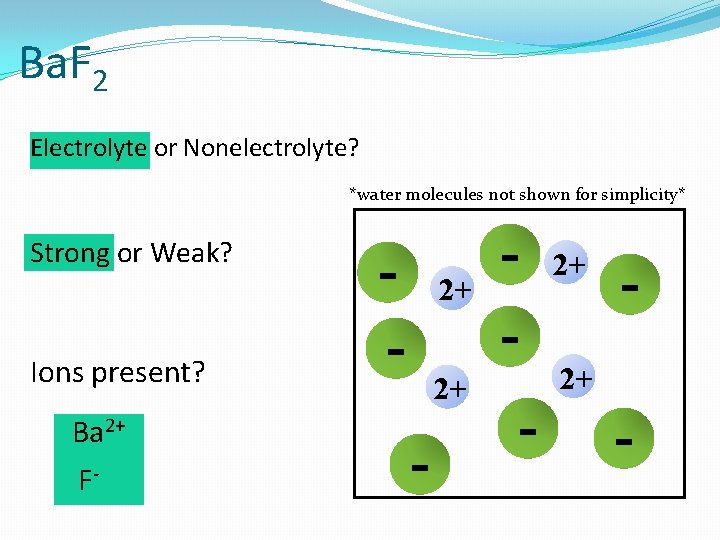
Ba. F 2 Electrolyte or Nonelectrolyte? *water molecules not shown for simplicity* Strong or Weak? Ions present? Ba 2+ F- - 2+ 2+ - - 2+ -

STOP

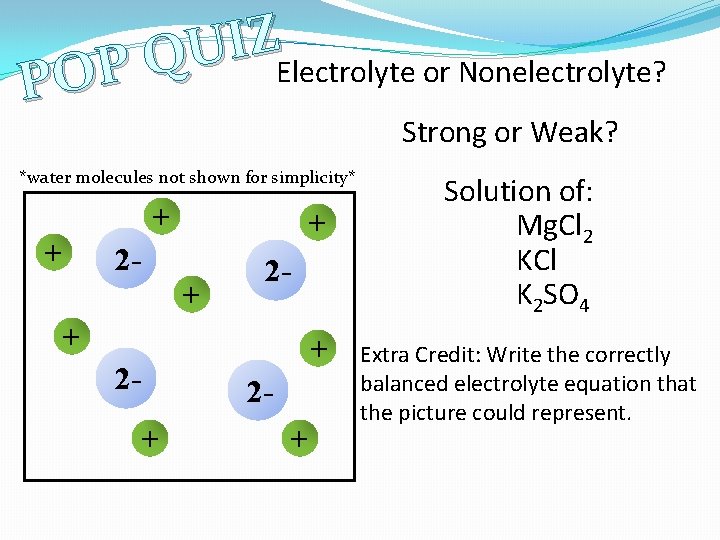
Z I U Q Electrolyte or Nonelectrolyte? P O P Strong or Weak? *water molecules not shown for simplicity* + + 2 - 2+ + Solution of: Mg. Cl 2 KCl K 2 SO 4 Extra Credit: Write the correctly balanced electrolyte equation that the picture could represent.

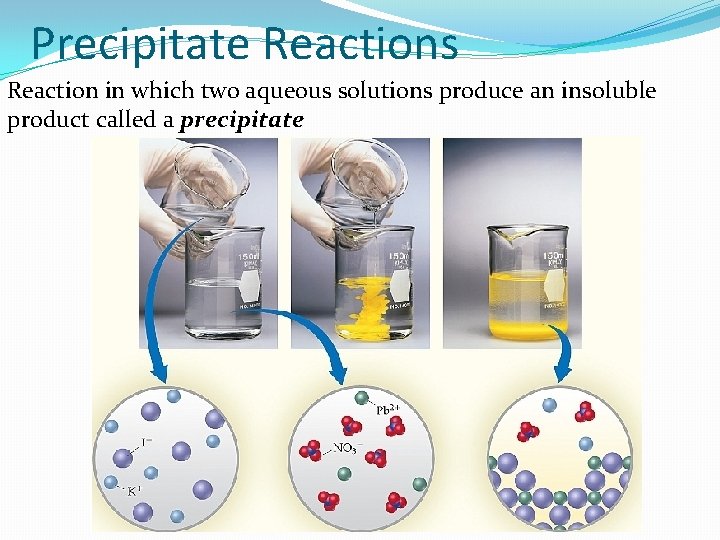
Precipitate Reactions Reaction in which two aqueous solutions produce an insoluble product called a precipitate

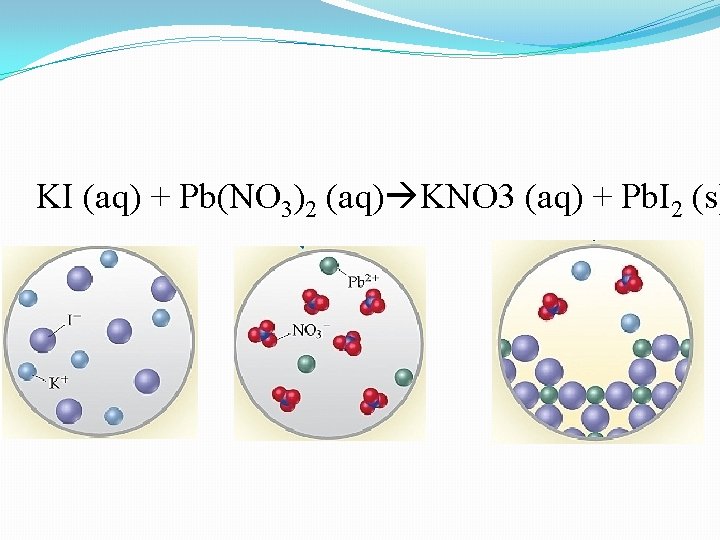
KI (aq) + Pb(NO 3)2 (aq) KNO 3 (aq) + Pb. I 2 (s)

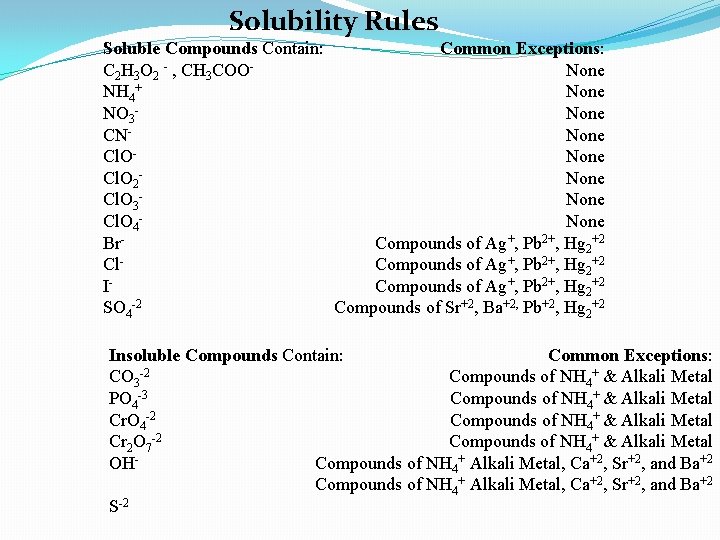
Solubility Rules Soluble Compounds Contain: Common Exceptions: C 2 H 3 O 2 - , CH 3 COONone NH 4+ None NO 3 None CNNone Cl. O 2 None Cl. O 3 None Cl. O 4 None Br. Compounds of Ag+, Pb 2+, Hg 2+2 Cl. Compounds of Ag+, Pb 2+, Hg 2+2 ICompounds of Ag+, Pb 2+, Hg 2+2 SO 4 -2 Compounds of Sr+2, Ba+2, Pb+2, Hg 2+2 Insoluble Compounds Contain: Common Exceptions: CO 3 -2 Compounds of NH 4+ & Alkali Metal PO 4 -3 Compounds of NH 4+ & Alkali Metal Cr. O 4 -2 Compounds of NH 4+ & Alkali Metal Cr 2 O 7 -2 Compounds of NH 4+ & Alkali Metal OHCompounds of NH 4+ Alkali Metal, Ca+2, Sr+2, and Ba+2 S-2

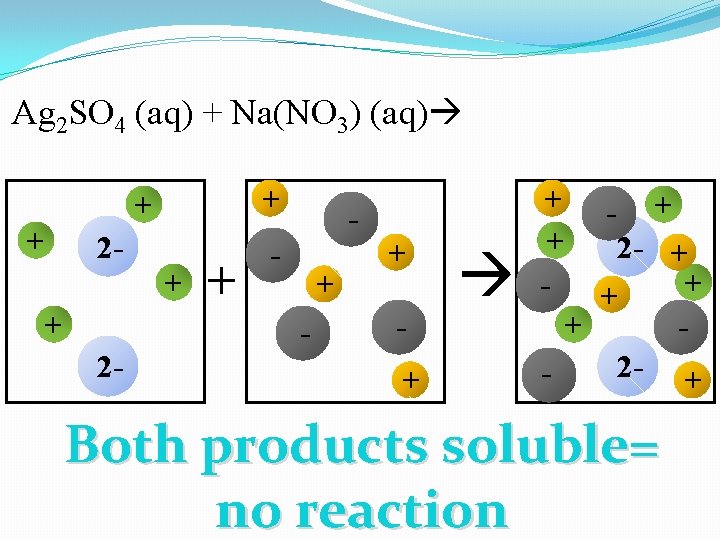
Ag 2 SO 4 (aq) + Na(NO 3) (aq) + + + 2 - + + - - + + + - + + 2 - + - Both products soluble= no reaction

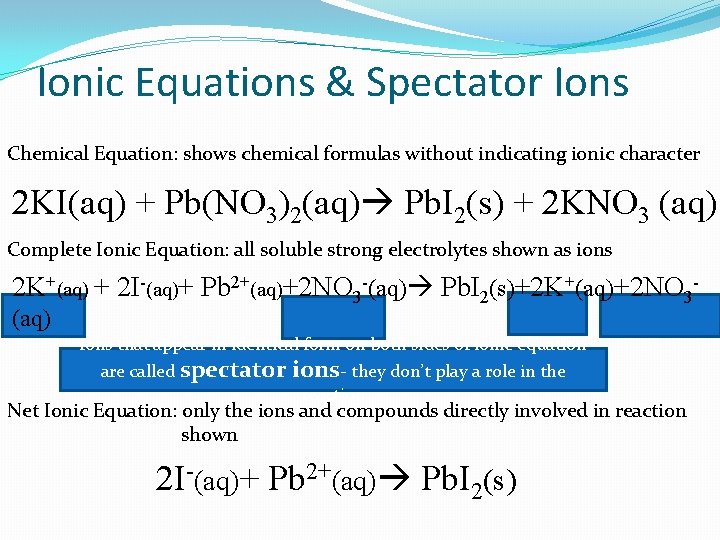
Ionic Equations & Spectator Ions Chemical Equation: shows chemical formulas without indicating ionic character 2 KI(aq) + Pb(NO 3)2(aq) Pb. I 2(s) + 2 KNO 3 (aq) Complete Ionic Equation: all soluble strong electrolytes shown as ions 2 K+(aq) + 2 I-(aq)+ Pb 2+(aq)+2 NO 3 -(aq) Pb. I 2(s)+2 K+(aq)+2 NO 3(aq) Ions that appear in identical form on both sides of ionic equation are called spectator ions- they don’t play a role in the reaction Net Ionic Equation: only the ions and compounds directly involved in reaction shown 2 I-(aq)+ Pb 2+(aq) Pb. I 2(s)

Which ions if any, are spectator ions in this reaction? Na. Cl(aq) + Ag. NO 3(aq) Ag. Cl(s) + Na. NO 3 (aq)

STOP

Concentration of Solutions Concentration: amount of solute dissolved in given quantity of solvent or quantity of solution

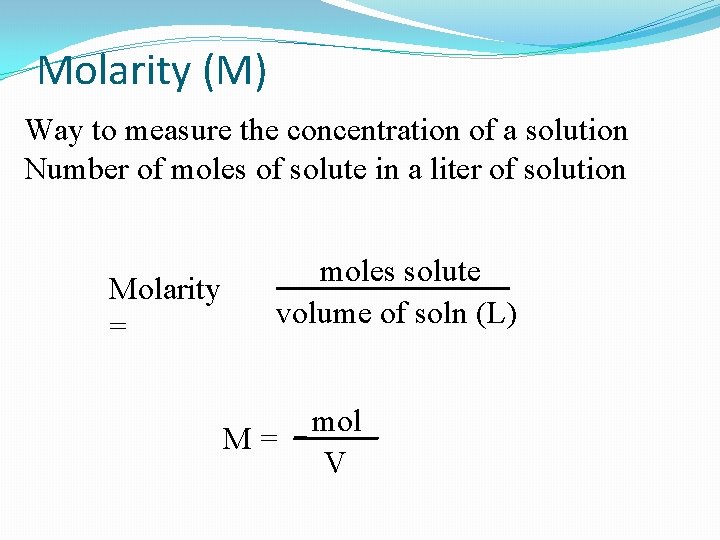
Molarity (M) Way to measure the concentration of a solution Number of moles of solute in a liter of solution Molarity = moles solute volume of soln (L) M= mol V

Practice! Calculate the molarity of a solution made by dissolving 23. 4 g of sodium sulfate in enough water to form 125 m. L of solution.

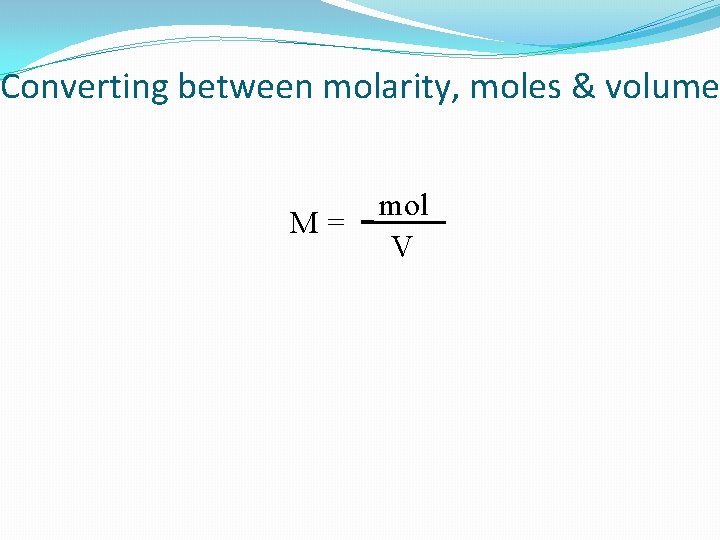
Converting between molarity, moles & volume M= mol V

Practice! A solution of HNO 3 has a concentration of 0. 200 M; how many moles does a 2. 0 L solution contain?

Making Solutions • Mass appropriate amount of solute • Add to container (volumetric flask, beaker, Erlenmeyer flask etc) • Add water, swirl to dissolve • Continue to add water until required volume of solution reached

Dilution Process of making a lower concentration solution from a higher concentrated solution. Add WATER

M= mol V Dilution **Since you’re not changing the amount of solute: ** Mol of solute before dilution = mol of solute after dilution M 1 V 1 =M 2 V 2

Practice! How many milliliters of 3. 0 M H 2 SO 4 are needed to make 450 m. L of 0. 10 M H 2 SO 4?