Solutions Solutions are uniform mixtures Solvent the substance

Solutions • Solutions are uniform mixtures. • Solvent: the substance present in the highest percentage • Solute: the dissolved substance, which is present in lesser amount • Aqueous solutions: solutions with water as the solvent Copyright © Houghton Mifflin Company. All rights reserved. 15 | 1

Types of Solutions • Solid in liquid (seawater). • Gas in liquid (carbonated water). • Liquid in liquid (antifreeze in water). • Solid in solid (zinc in copper = brass). Copyright © Houghton Mifflin Company. All rights reserved. 15 | 2
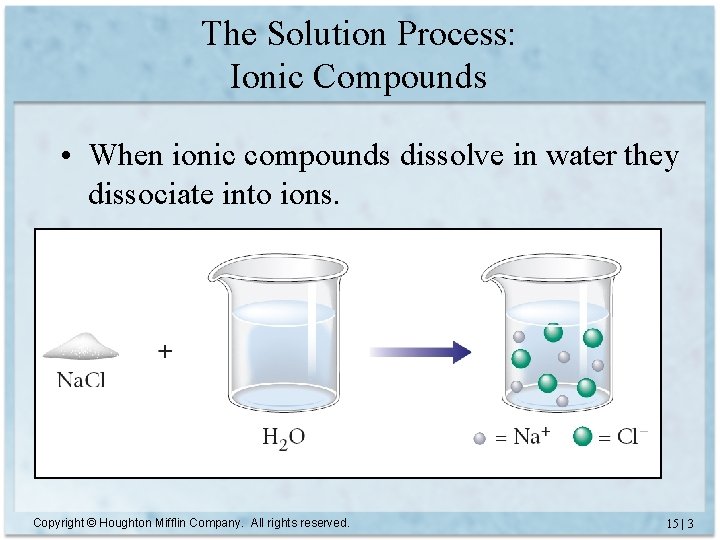
The Solution Process: Ionic Compounds • When ionic compounds dissolve in water they dissociate into ions. Copyright © Houghton Mifflin Company. All rights reserved. 15 | 3
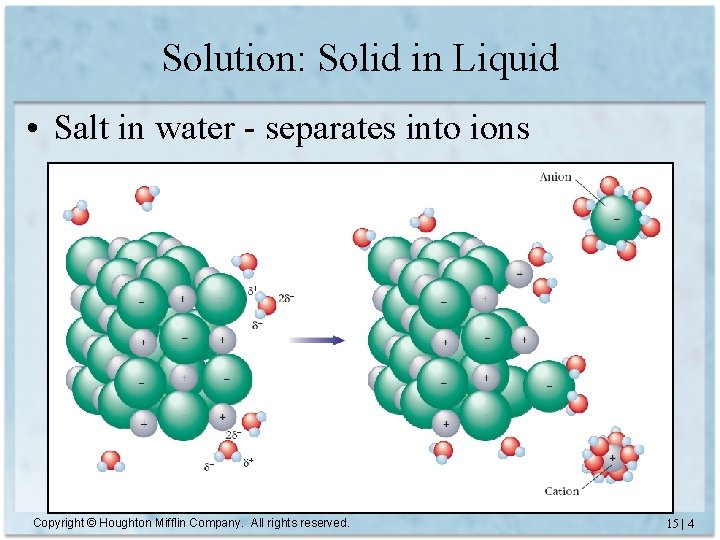
Solution: Solid in Liquid • Salt in water - separates into ions Copyright © Houghton Mifflin Company. All rights reserved. 15 | 4

Solubility • When one substance dissolves in another, it is said to be soluble. – Salt is soluble in water • When one substance does not dissolve in another, it is said to be insoluble. – Oil is insoluble in water Copyright © Houghton Mifflin Company. All rights reserved. 15 | 5
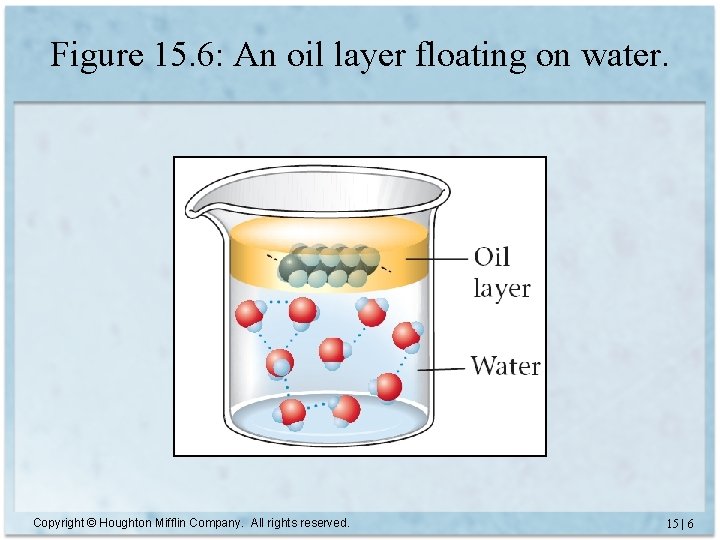
Figure 15. 6: An oil layer floating on water. Copyright © Houghton Mifflin Company. All rights reserved. 15 | 6

How concentrated? • A concentrated solution has a high proportion of solute to solution. • A dilute solution has a low proportion of solute to solution. Copyright © Houghton Mifflin Company. All rights reserved. 15 | 7

How concentrated? (cont. ) • A saturated solution has the maximum amount of solute that will dissolve in the solvent. • An unsaturated solution has less than the saturation limit. Copyright © Houghton Mifflin Company. All rights reserved. 15 | 8

How much? • Concentration: the amount of solute in a given amount of solution Copyright © Houghton Mifflin Company. All rights reserved. 15 | 9

Solution Concentration- Molarity • Moles of solute per 1 liter of solution • If a sugar solution concentration is 2. 0 M, 1 liter of solution contains 2. 0 moles of sugar, 2 liters = 4. 0 moles sugar, 0. 5 liters = 1. 0 mole sugar, etc. moles of solute molarity = liters of solution Copyright © Houghton Mifflin Company. All rights reserved. 15 | 10

Molarity of ions • Ca. Cl 2(aq) = Ca+2(aq) + 2 Cl-1(aq) • A 1. 0 M Ca. Cl 2(aq) solution contains 1. 0 moles of Ca. Cl 2 in each liter of solution • Because each Ca. Cl 2 dissociates to one Ca+2, 1. 0 M Ca. Cl 2 = 1. 0 M Ca+2 • Because each Ca. Cl 2 dissociates to 2 Cl-1, 1. 0 M Ca. Cl 2 = 2. 0 M Cl-1 Copyright © Houghton Mifflin Company. All rights reserved. 15 | 11

Dilution • Dilution: adding solvent to decrease the concentration of a solution • The amount of solute stays the same, but the concentration decreases. Copyright © Houghton Mifflin Company. All rights reserved. 15 | 12

Dilution (cont. ) • Dilution Formula M 1 x V 1 = M 2 x V 2 # Moles/L · # L = # moles – In dilution we take a certain number of moles of solute and dilute to a bigger volume. Copyright © Houghton Mifflin Company. All rights reserved. 15 | 13

Copyright © Houghton Mifflin Company. All rights reserved. 15 | 14
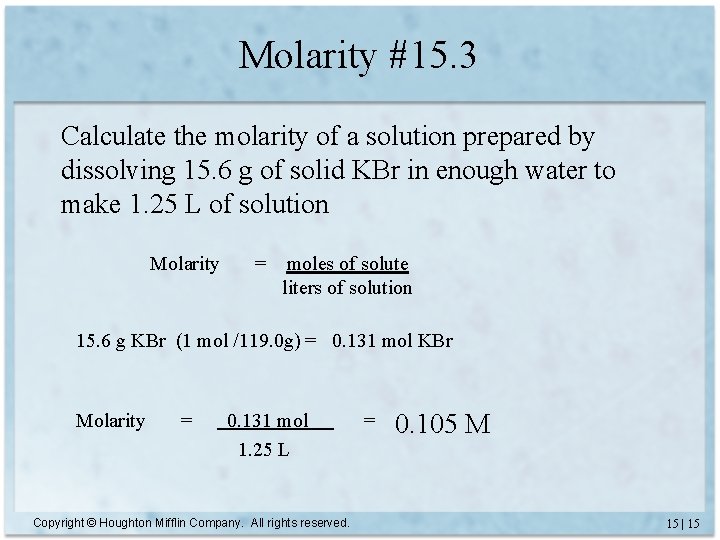
Molarity #15. 3 Calculate the molarity of a solution prepared by dissolving 15. 6 g of solid KBr in enough water to make 1. 25 L of solution Molarity = moles of solute liters of solution 15. 6 g KBr (1 mol /119. 0 g) = 0. 131 mol KBr Molarity = 0. 131 mol 1. 25 L Copyright © Houghton Mifflin Company. All rights reserved. = 0. 105 M 15 | 15
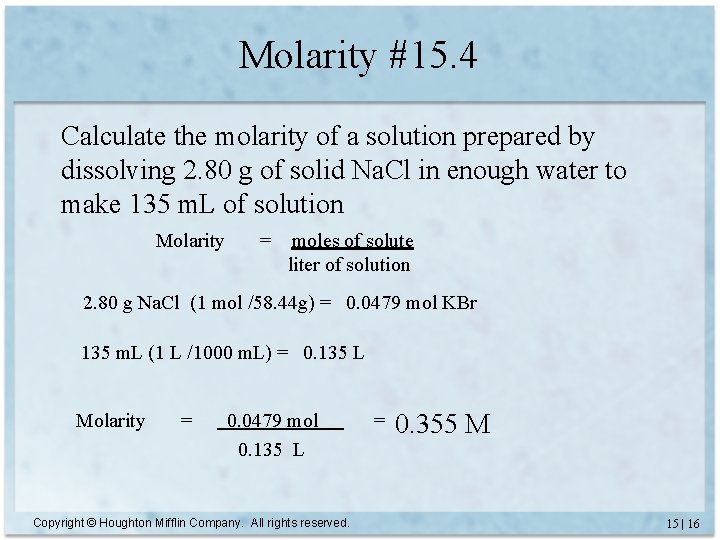
Molarity #15. 4 Calculate the molarity of a solution prepared by dissolving 2. 80 g of solid Na. Cl in enough water to make 135 m. L of solution Molarity = moles of solute liter of solution 2. 80 g Na. Cl (1 mol /58. 44 g) = 0. 0479 mol KBr 135 m. L (1 L /1000 m. L) = 0. 135 L Molarity = 0. 0479 mol 0. 135 L Copyright © Houghton Mifflin Company. All rights reserved. = 0. 355 M 15 | 16
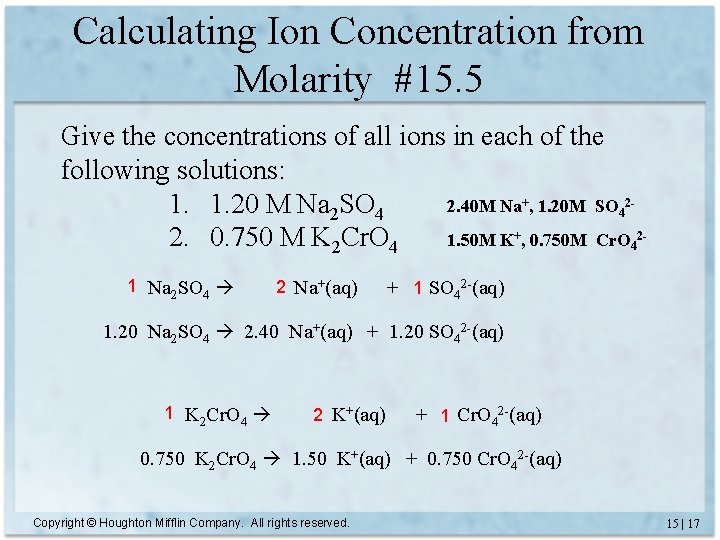
Calculating Ion Concentration from Molarity #15. 5 Give the concentrations of all ions in each of the following solutions: 2. 40 M Na+, 1. 20 M SO 421. 1. 20 M Na 2 SO 4 1. 50 M K+, 0. 750 M Cr. O 422. 0. 750 M K 2 Cr. O 4 1 Na 2 SO 4 2 Na+(aq) + 1 SO 42 -(aq) 1. 20 Na 2 SO 4 2. 40 Na+(aq) + 1. 20 SO 42 -(aq) 1 K 2 Cr. O 4 2 K+(aq) + 1 Cr. O 42 -(aq) 0. 750 K 2 Cr. O 4 1. 50 K+(aq) + 0. 750 Cr. O 42 -(aq) Copyright © Houghton Mifflin Company. All rights reserved. 15 | 17
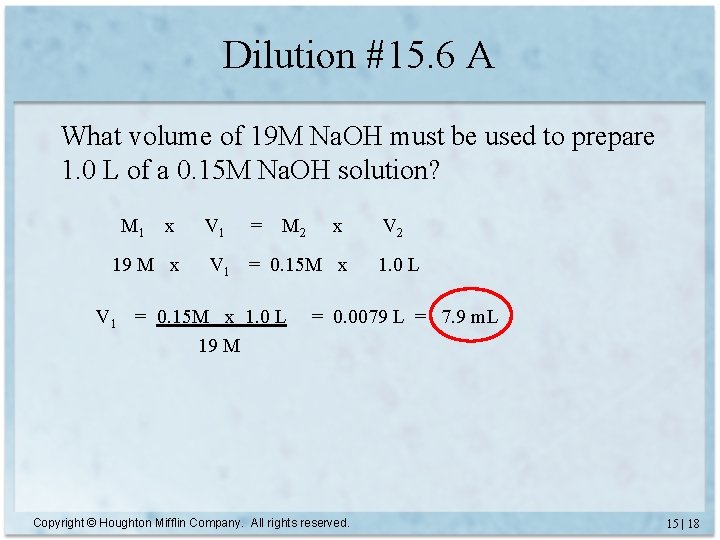
Dilution #15. 6 A What volume of 19 M Na. OH must be used to prepare 1. 0 L of a 0. 15 M Na. OH solution? M 1 x 19 M x V 1 = M 2 x V 1 = 0. 15 M x 1. 0 L 19 M V 2 1. 0 L = 0. 0079 L = 7. 9 m. L Copyright © Houghton Mifflin Company. All rights reserved. 15 | 18
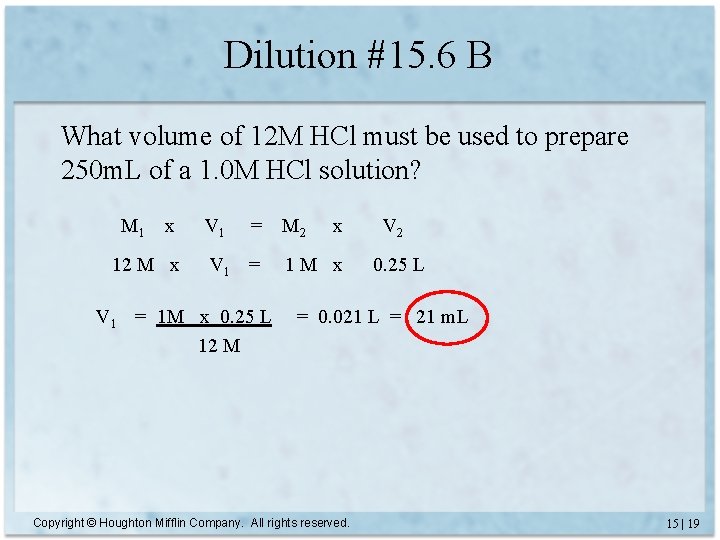
Dilution #15. 6 B What volume of 12 M HCl must be used to prepare 250 m. L of a 1. 0 M HCl solution? M 1 x 12 M x V 1 = 1 M x 0. 25 L 12 M M 2 x 1 M x V 2 0. 25 L = 0. 021 L = 21 m. L Copyright © Houghton Mifflin Company. All rights reserved. 15 | 19
- Slides: 19