Solutions Solute Solvents I What is a solution















- Slides: 46

Solutions Solute Solvents

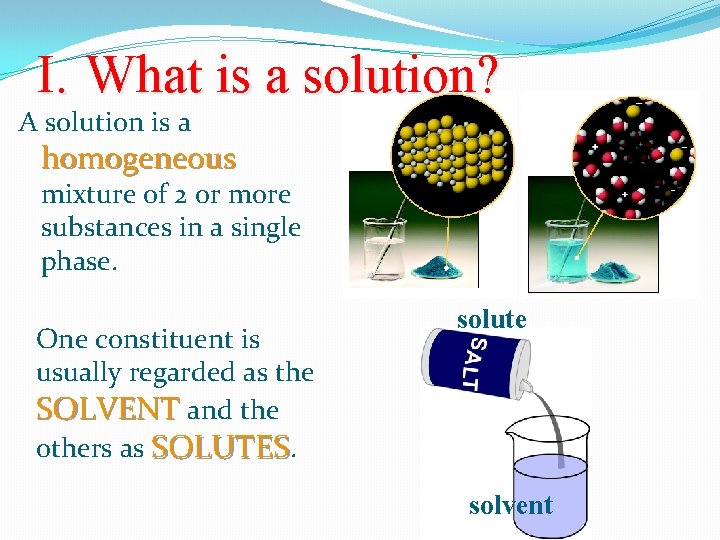
I. What is a solution? A solution is a homogeneous mixture of 2 or more substances in a single phase. One constituent is usually regarded as the SOLVENT and the others as SOLUTES. solute solvent

Parts of a Solution �SOLUTE – the part of a solution that is being dissolved (usually the lesser amount) �SOLVENT – the part of a solution that dissolves the solute (usually the greater amount) �Solute + Solvent = Solution Solvent Solute

Nature of Solutes in Solutions l Spread evenly throughout the solution l Cannot be separated by filtration l Can be separated by evaporation l Not visible, solution appears transparent l May give a color to the solution 4

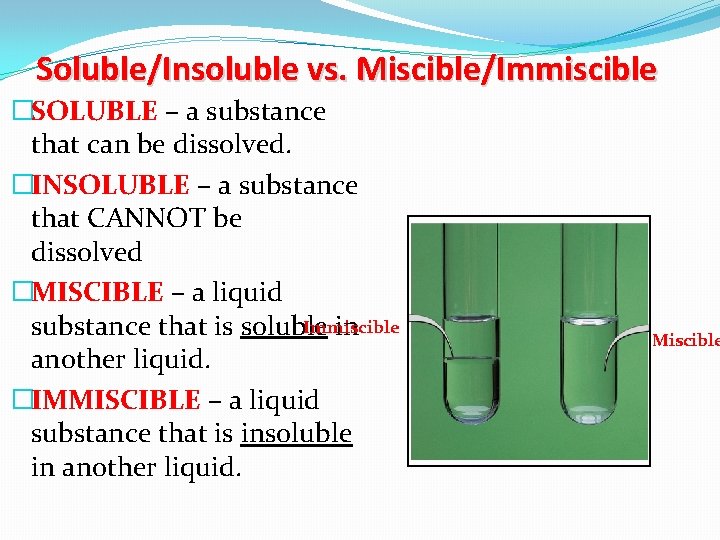
Soluble/Insoluble vs. Miscible/Immiscible �SOLUBLE – a substance that can be dissolved. �INSOLUBLE – a substance that CANNOT be dissolved �MISCIBLE – a liquid Immiscible substance that is soluble in another liquid. �IMMISCIBLE – a liquid substance that is insoluble in another liquid. Miscible

Examples Salt added to water: The solute is the salt The solvent is the water This makes a solution The salt is soluble as it has dissolved Flour added to water. Stirring it makes it go cloudy, but after a while all the flour grains sink to the bottom: The flours has not dissolved This is because flour is insoluble

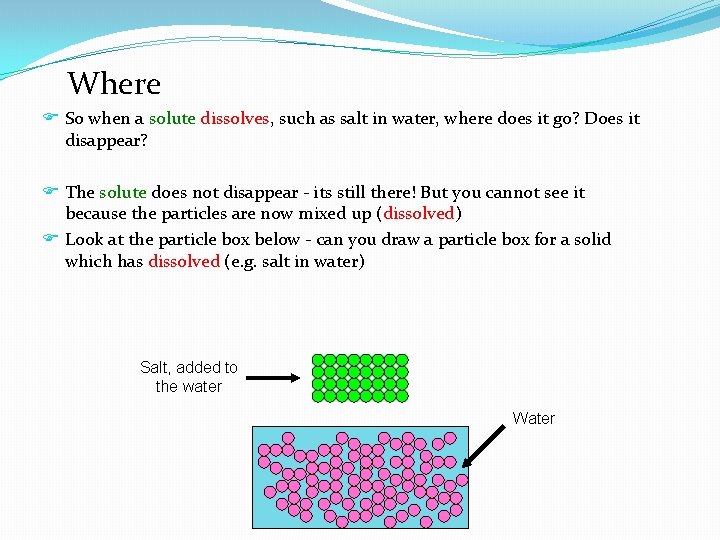
Where F So when a solute dissolves, such as salt in water, where does it go? Does it disappear? F The solute does not disappear - its still there! But you cannot see it because the particles are now mixed up (dissolved) F Look at the particle box below - can you draw a particle box for a solid which has dissolved (e. g. salt in water) Salt, added to the water Water

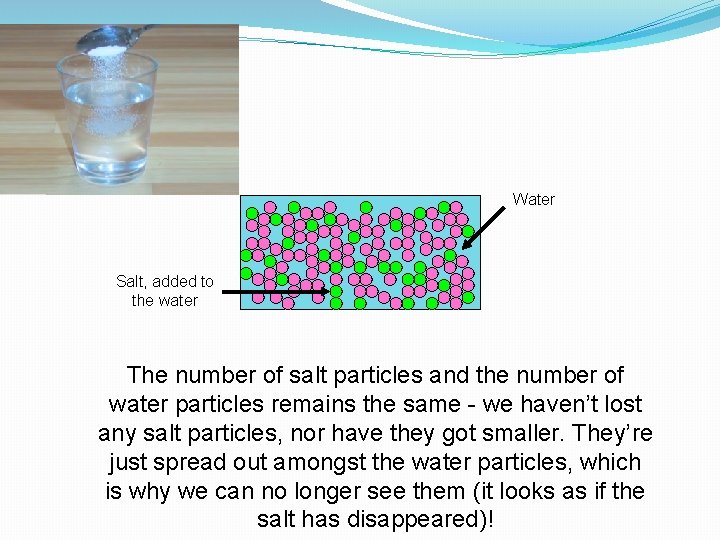
Water Salt, added to the water The number of salt particles and the number of water particles remains the same - we haven’t lost any salt particles, nor have they got smaller. They’re just spread out amongst the water particles, which is why we can no longer see them (it looks as if the salt has disappeared)!

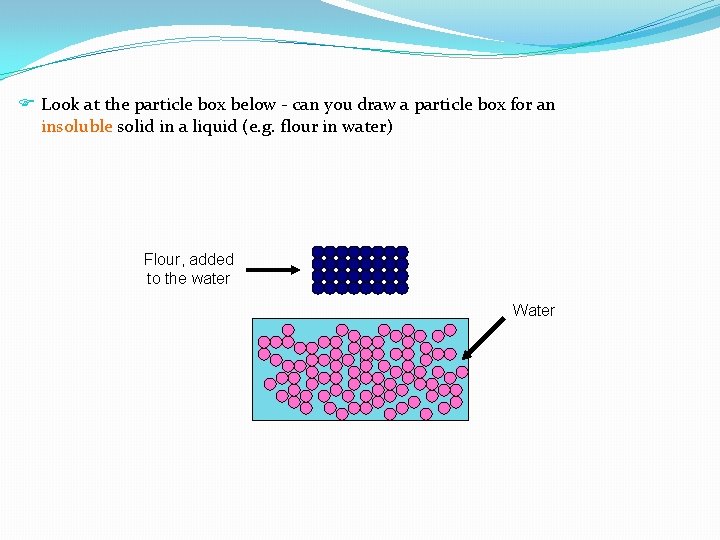
F Look at the particle box below - can you draw a particle box for an insoluble solid in a liquid (e. g. flour in water) Flour, added to the water Water

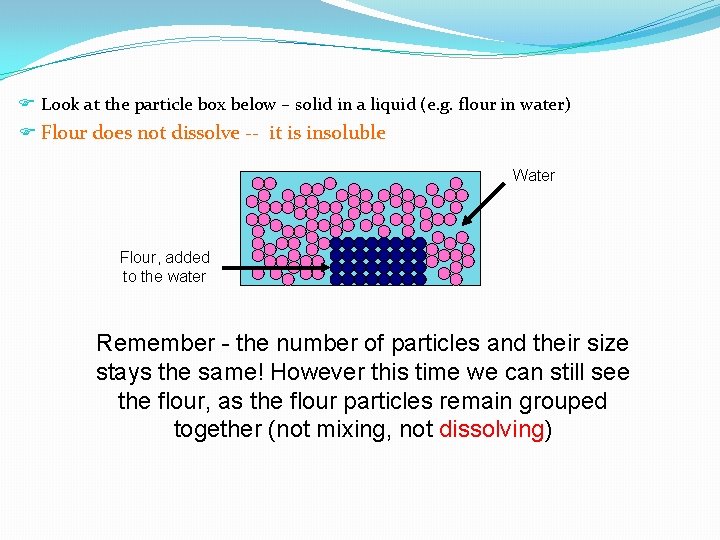
F Look at the particle box below – solid in a liquid (e. g. flour in water) F Flour does not dissolve -- it is insoluble Water Flour, added to the water Remember - the number of particles and their size stays the same! However this time we can still see the flour, as the flour particles remain grouped together (not mixing, not dissolving)

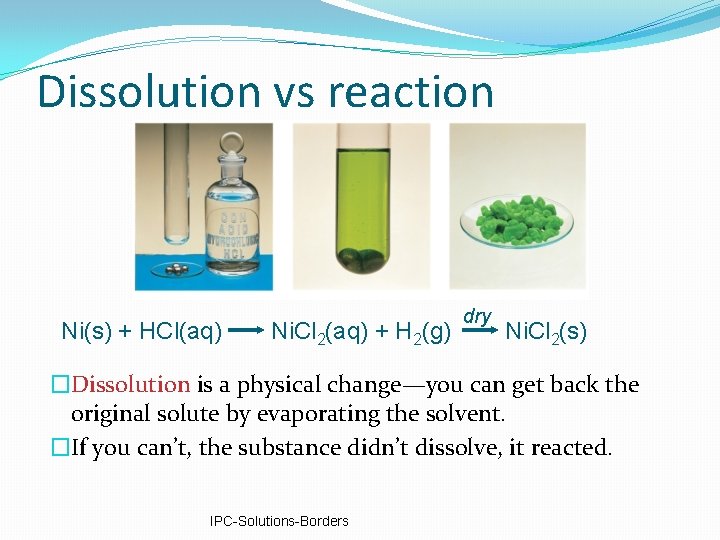
Dissolution vs reaction Ni(s) + HCl(aq) Ni. Cl 2(aq) + H 2(g) dry Ni. Cl 2(s) �Dissolution is a physical change—you can get back the original solute by evaporating the solvent. �If you can’t, the substance didn’t dissolve, it reacted. IPC-Solutions-Borders

Types of Solutions 1. 2. 3. Gaseous solutions Liquid solutions (most common) Solid solutions--alloys

Types of Solutions Examples Gas in a Liquid in a Liquid Solids in Solids Non-Examples salad soil Air Soda Gasoline Sea Water Brass water

Solutions How does a solid dissolve into a liquid? IPC-Solutions-Borders

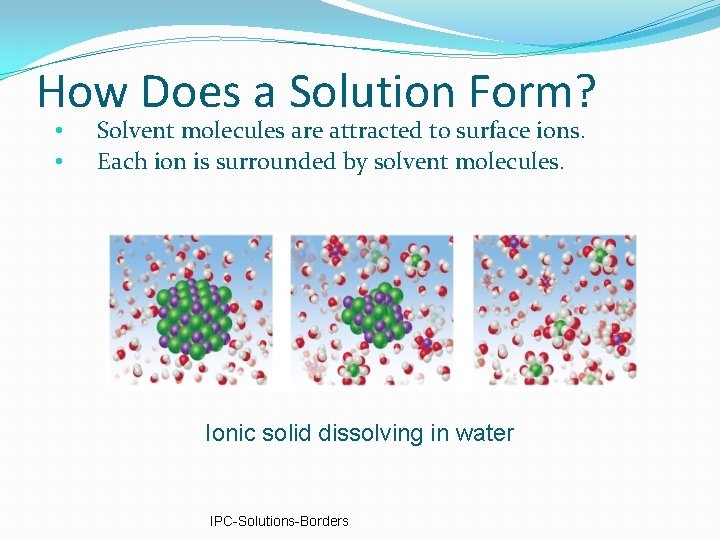
How Does a Solution Form? • • Solvent molecules are attracted to surface ions. Each ion is surrounded by solvent molecules. Ionic solid dissolving in water IPC-Solutions-Borders

The amount of solute that dissolves can vary �Concentration: The amount of solute dissolved in a solvent at a given temperature. �Examples: �Hot chocolate…the more powdered mix you add the higher the concentration of chocolate �Lemonade…the more frozen lemon concentrate or powdered mix you add the more tart the drink becomes www. thesunblog. com

www. seroundtable. com Degrees of Concentration �Dilute or Unsaturated: a solution has a low concentration of solute �Saturated: a solution that contains the maximum amount of solute that can be dissolved into the solvent at a given temperature. �Supersaturated: a solution can contain more solute than normal by raising the temperature of the solvent.

Degree of saturation �Unsaturated Solution Ø Less than the maximum amount of solute for that temperature is dissolved in the solvent. Ø No solid remains in flask.

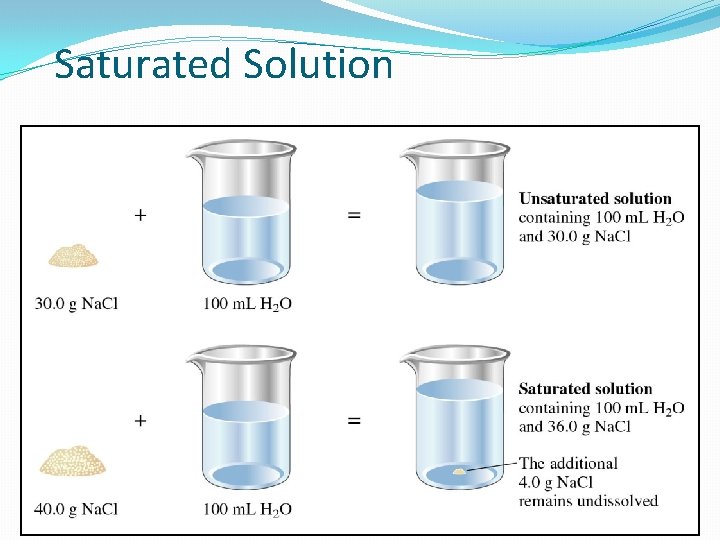
Degree of saturation �Saturated solution Ø Solvent holds as much solute as is possible at that temperature. Ø Undissolved solid remains in flask. Ø Dissolved solute is in dynamic equilibrium with solid solute particles.

Saturated Solution

Degree of saturation �Supersaturated Solution Ø Solvent holds more solute than is normally possible at that temperature. Ø These solutions are unstable; crystallization can often be caused by adding a “seed crystal” or scratching the side of the flask.

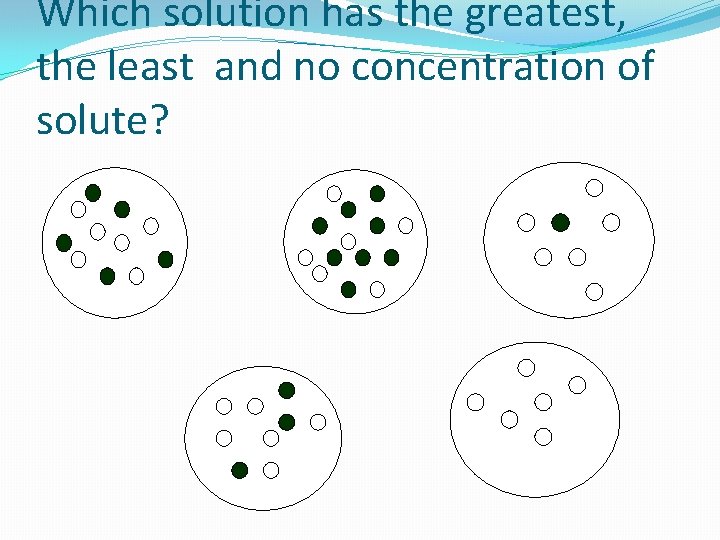
Which solution has the greatest, the least and no concentration of solute?

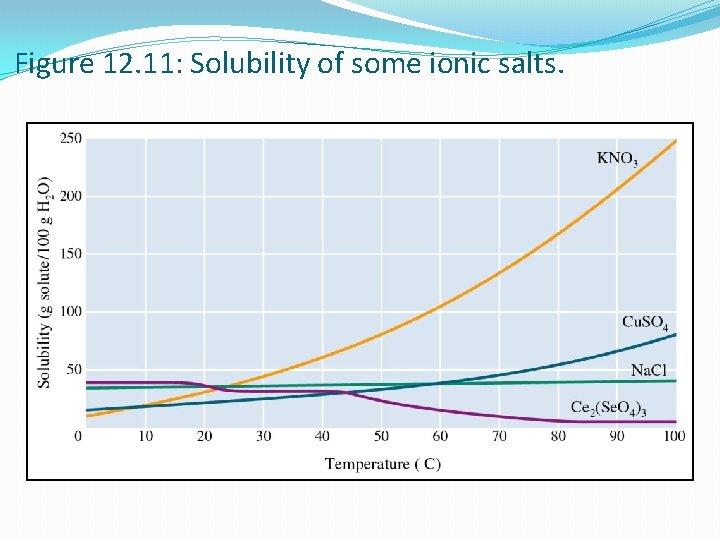
Figure 12. 11: Solubility of some ionic salts.

Solubility of a Solute What are ways that you make a solute dissolve faster in water? 1) Increase the temperature. 2) Increase the pressure 3) Crush or use smaller size solute particles. 4) Stir the solutions.

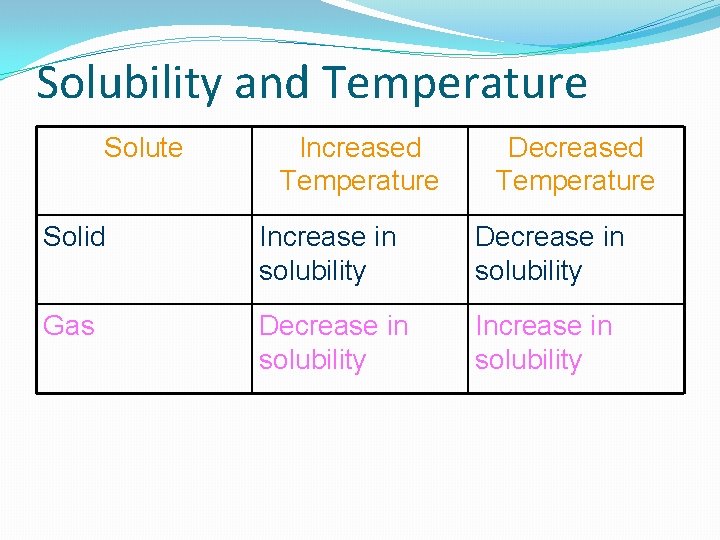
Solubility and Temperature Solute Increased Temperature Decreased Temperature Solid Increase in solubility Decrease in solubility Gas Decrease in solubility Increase in solubility

Gases in Solution Increasing pressure above solution forces more gas to dissolve. �Sudden release of pressure from a carbonated beverage.

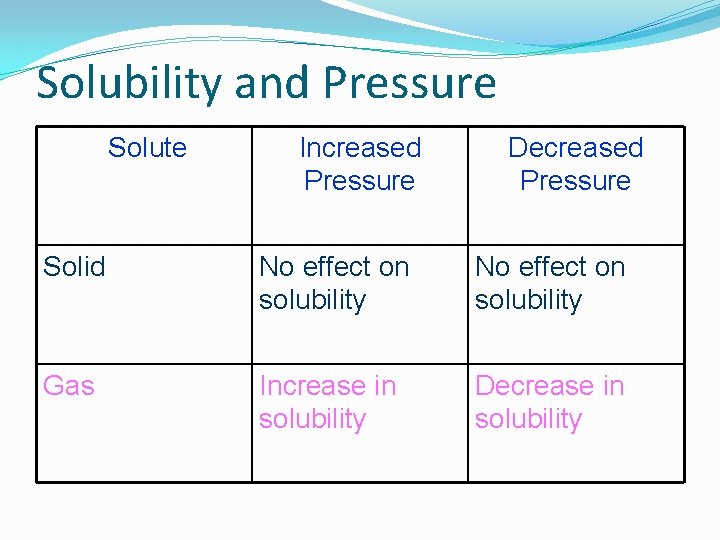
Solubility and Pressure Solute Increased Pressure Decreased Pressure Solid No effect on solubility Gas Increase in solubility Decrease in solubility

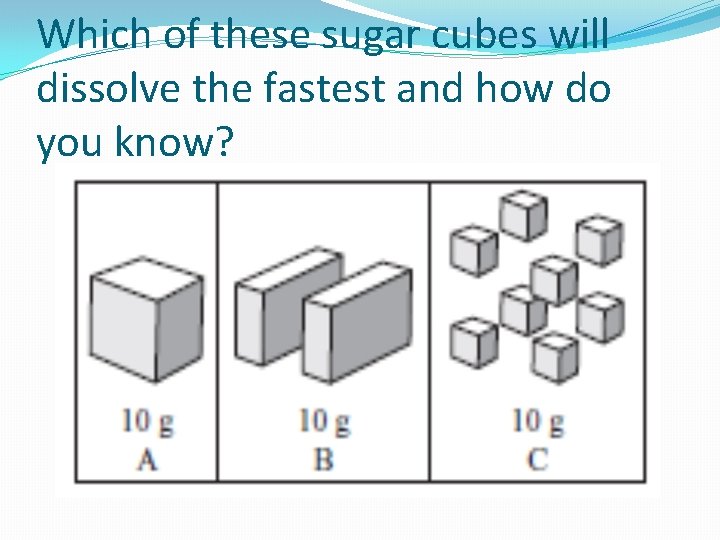
Which of these sugar cubes will dissolve the fastest and how do you know?

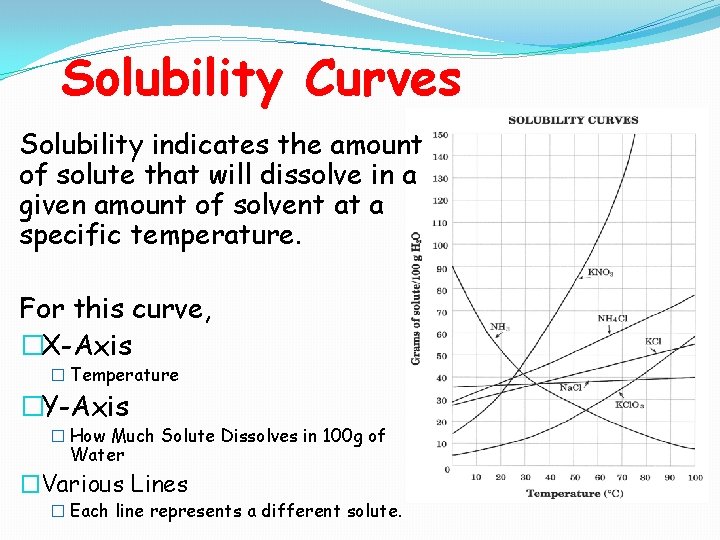
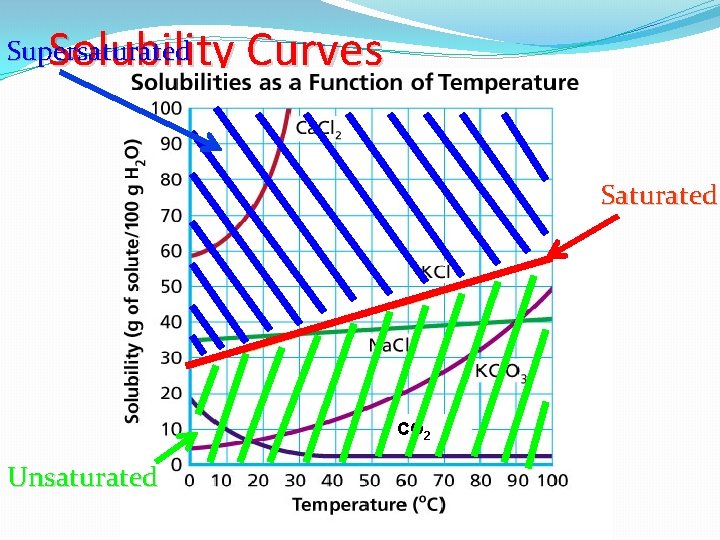
Solubility Curves Solubility indicates the amount of solute that will dissolve in a given amount of solvent at a specific temperature. For this curve, �X-Axis � Temperature �Y-Axis � How Much Solute Dissolves in 100 g of Water �Various Lines � Each line represents a different solute.

Solubility Curves Supersaturated Saturated CO 2 Unsaturated

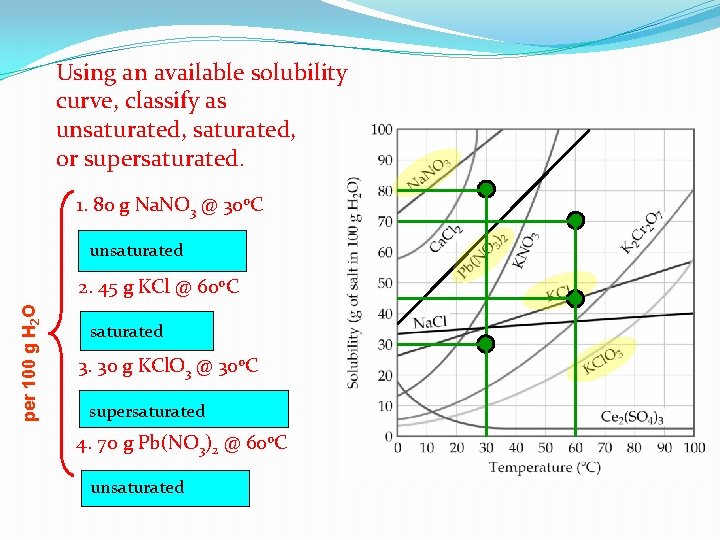
Using an available solubility curve, classify as unsaturated, or supersaturated. 1. 80 g Na. NO 3 @ 30 o. C unsaturated per 100 g H 2 O 2. 45 g KCl @ 60 o. C saturated 3. 30 g KCl. O 3 @ 30 o. C supersaturated 4. 70 g Pb(NO 3)2 @ 60 o. C unsaturated

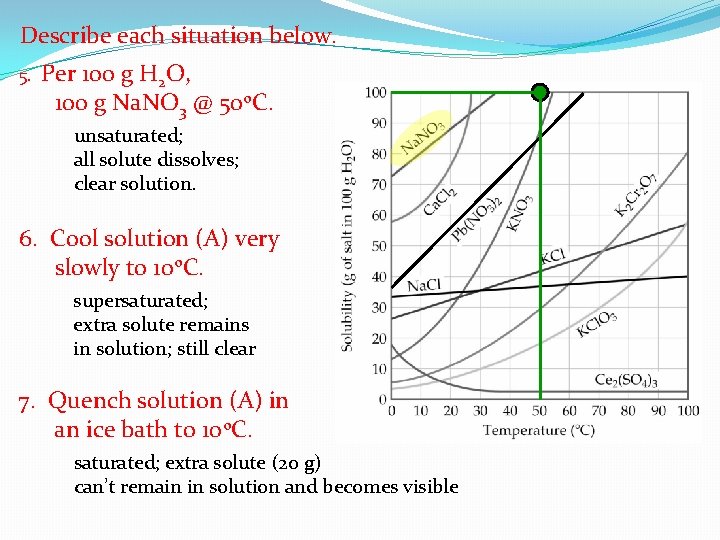
Describe each situation below. 5. Per 100 g H 2 O, 100 g Na. NO 3 @ 50 o. C. unsaturated; all solute dissolves; clear solution. 6. Cool solution (A) very slowly to 10 o. C. supersaturated; extra solute remains in solution; still clear 7. Quench solution (A) in an ice bath to 10 o. C. saturated; extra solute (20 g) can’t remain in solution and becomes visible

How to use a solubility graph? A. IDENTIFYING A SUBSTANCE ( given the solubility in g/100 cm 3 of water and the temperature) • Look for the intersection of the solubility and temperature.

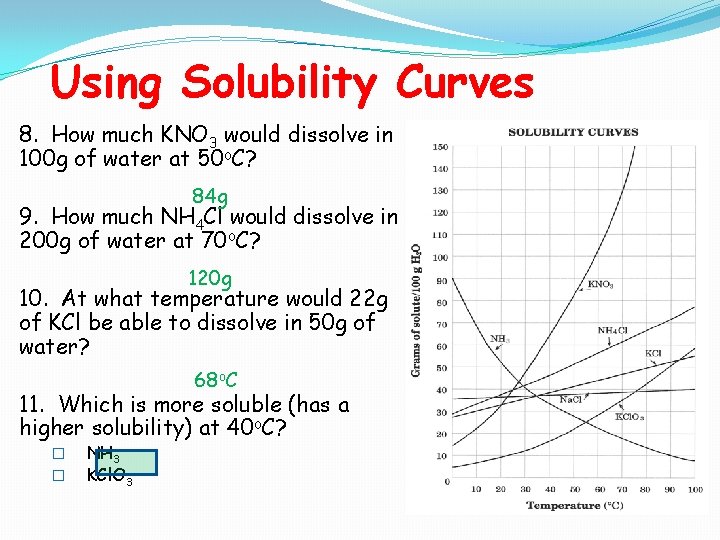
Using Solubility Curves 8. How much KNO 3 would dissolve in 100 g of water at 50 o. C? 84 g 9. How much NH 4 Cl would dissolve in 200 g of water at 70 o. C? 120 g 10. At what temperature would 22 g of KCl be able to dissolve in 50 g of water? 68 o. C 11. Which is more soluble (has a higher solubility) at 40 o. C? � � NH 3 KCl. O 3

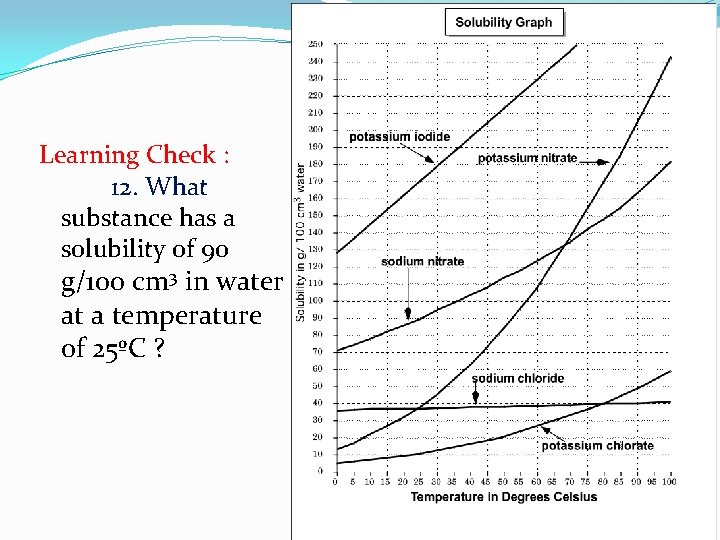
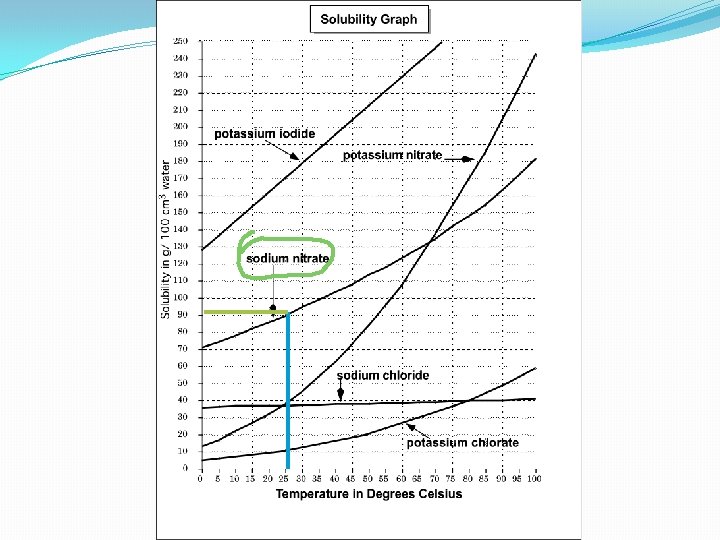
Learning Check : 12. What substance has a solubility of 90 g/100 cm 3 in water at a temperature of 25ºC ?


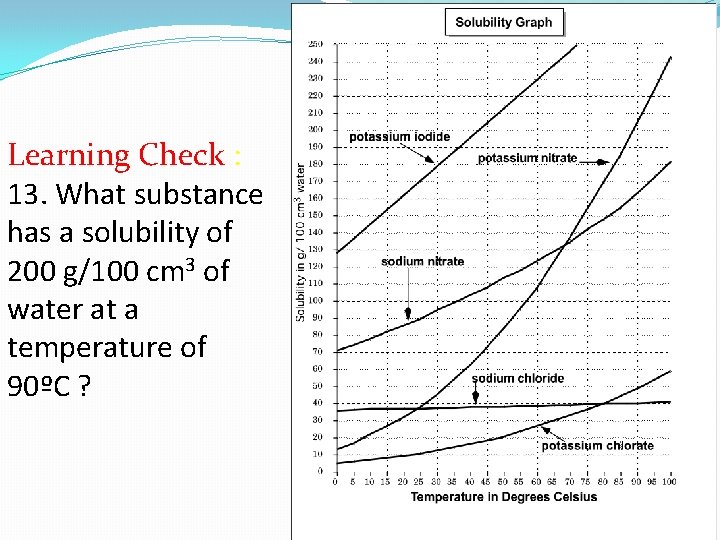
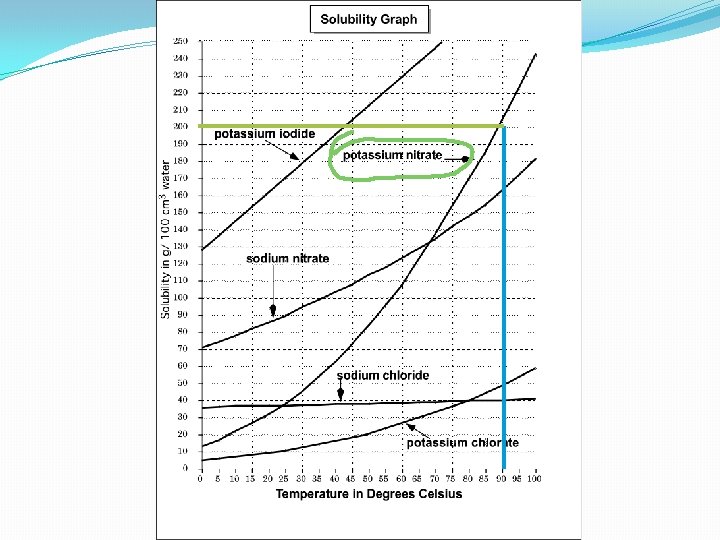
Learning Check : 13. What substance has a solubility of 200 g/100 cm 3 of water at a temperature of 90ºC ?


Look for the temperature or solubility • Locate the solubility curve needed and see for a given temperature, which solubility it lines up with and visa versa.

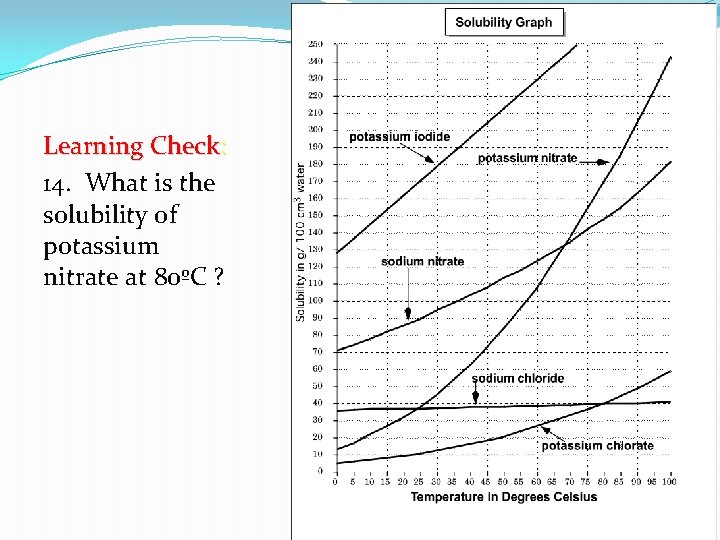
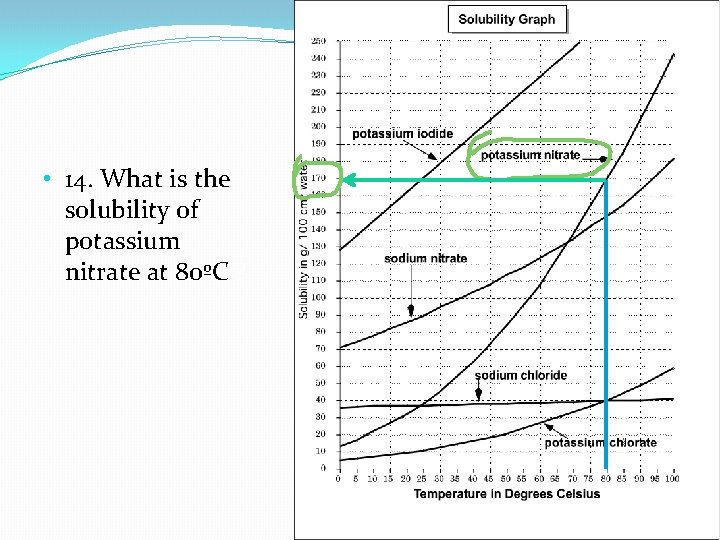
Learning Check: 14. What is the solubility of potassium nitrate at 80ºC ?

• 14. What is the solubility of potassium nitrate at 80ºC ?

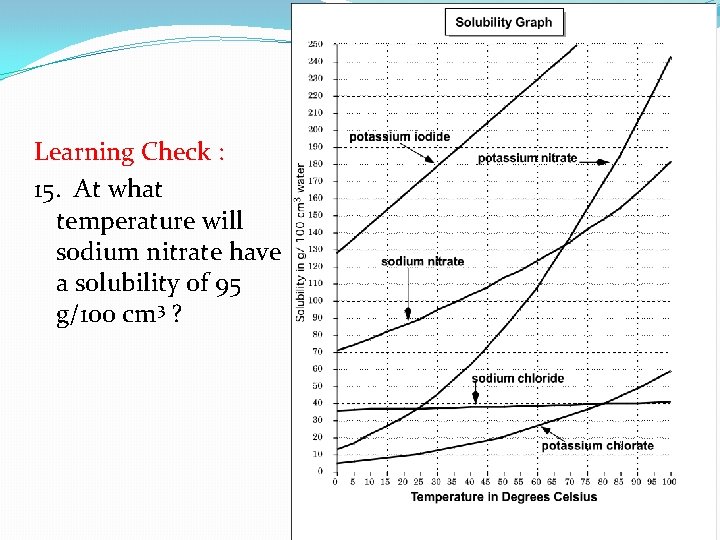
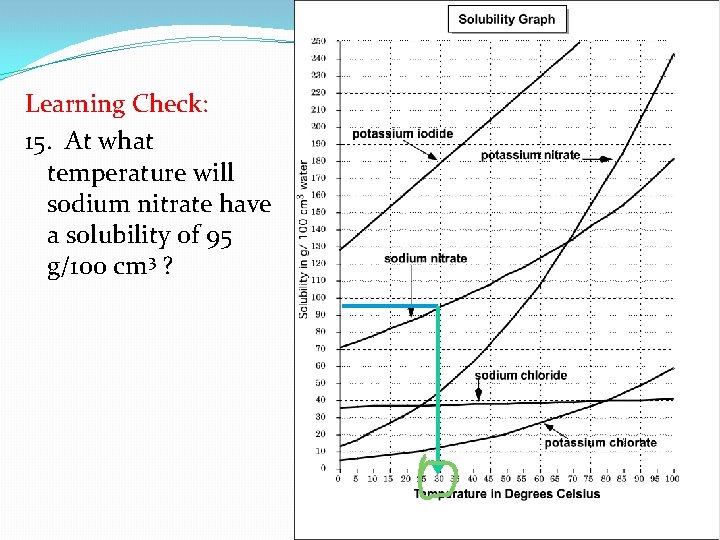
Learning Check : 15. At what temperature will sodium nitrate have a solubility of 95 g/100 cm 3 ?

Learning Check: 15. At what temperature will sodium nitrate have a solubility of 95 g/100 cm 3 ?

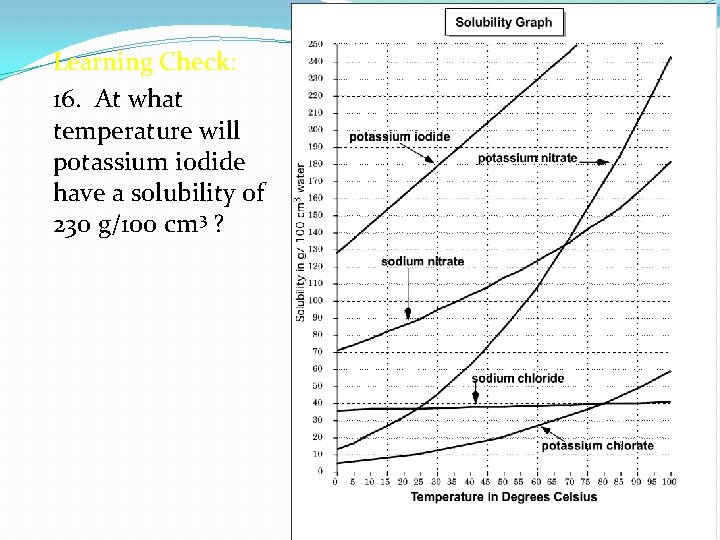
Learning Check: 16. At what temperature will potassium iodide have a solubility of 230 g/100 cm 3 ?

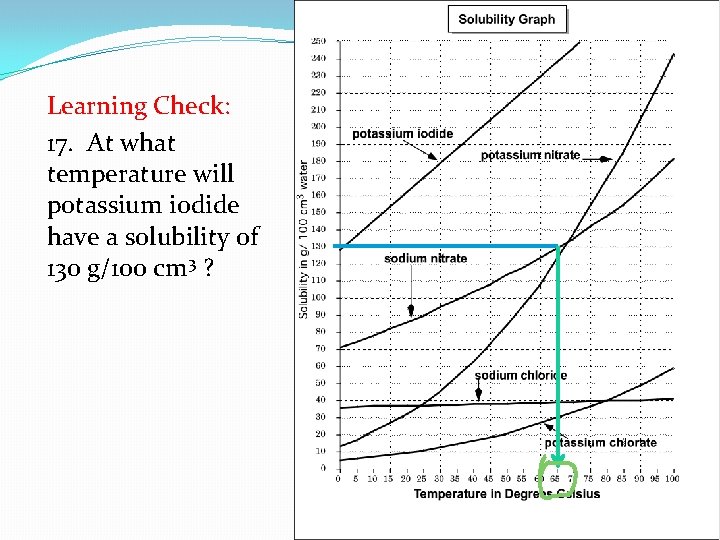
Learning Check: 17. At what temperature will potassium iodide have a solubility of 130 g/100 cm 3 ?

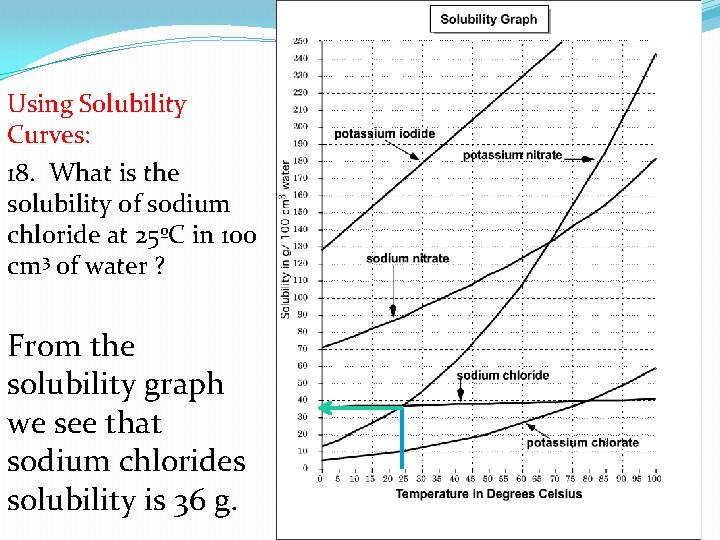
Using Solubility Curves: 18. What is the solubility of sodium chloride at 25ºC in 100 cm 3 of water ? From the solubility graph we see that sodium chlorides solubility is 36 g.