Solutions Partial Chapter 12 Vocabulary a homogeneous mixture

Solutions (Partial) Chapter 12

Vocabulary • _____: a homogeneous mixture of two or more substances in a single phase • ______: the dissolving medium in a solution; present in the greatest quantity • _____: the substance dissolved in a solution; present in a lesser quantity • Solutions can be liquid or solid or gaseous phases.
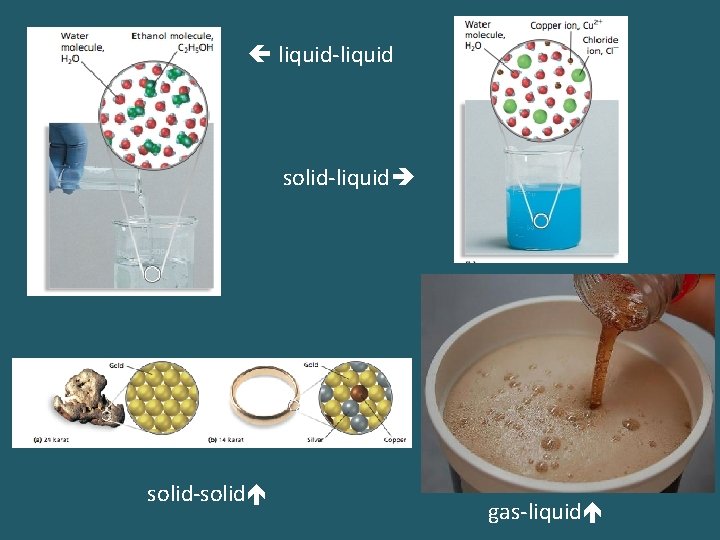
liquid-liquid solid-liquid solid-solid gas-liquid

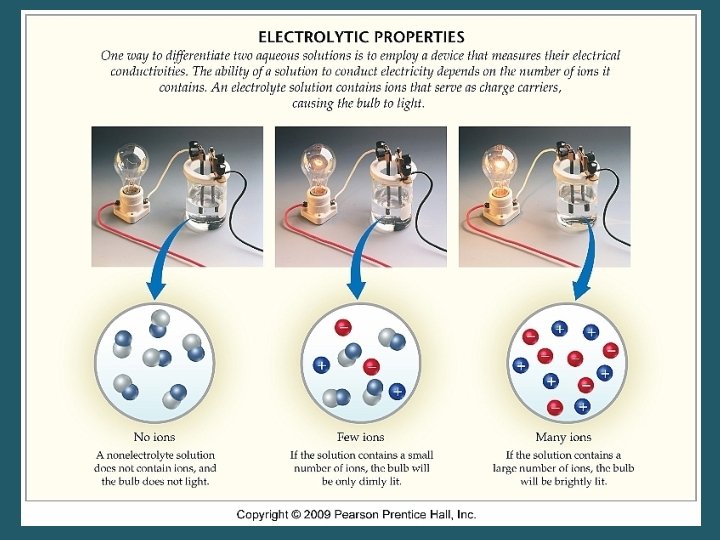
Vocabulary • _______- solutions consisting of solutes that will conduct electric current. • _______- solutions consisting of solutes that DO NOT conduct electric current.
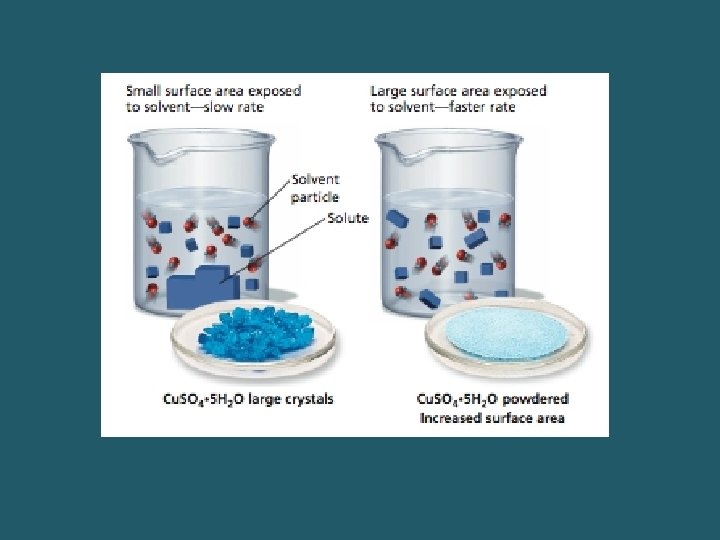
Factors Affecting the Rate of Dissolution 1. _______: greater surface area speeds up the rate 2. _____: shaking or stirring speeds up the rate 3. _______: higher temperatures usually favor a higher rate. – Common exception- gases. Then a lower temperature speeds up the rate
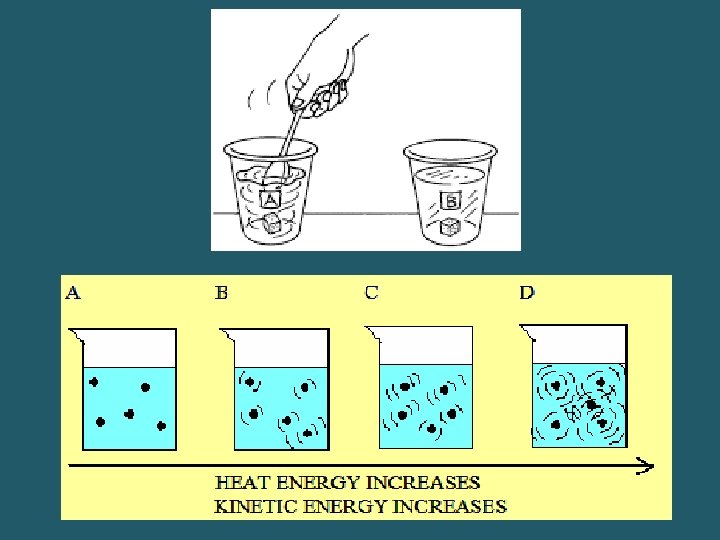

Vocabulary • _______- solutions that have the maximum amount of solute dissolved at a given set of conditions • ___________- solutions that DO NOT have the maximum amount of solute dissolved at a given set of conditions

Vocabulary • _____- liquids that combine freely (dissolve) with one another • ________- liquids that DO NOT combine freely (DON’T dissolve) with one another

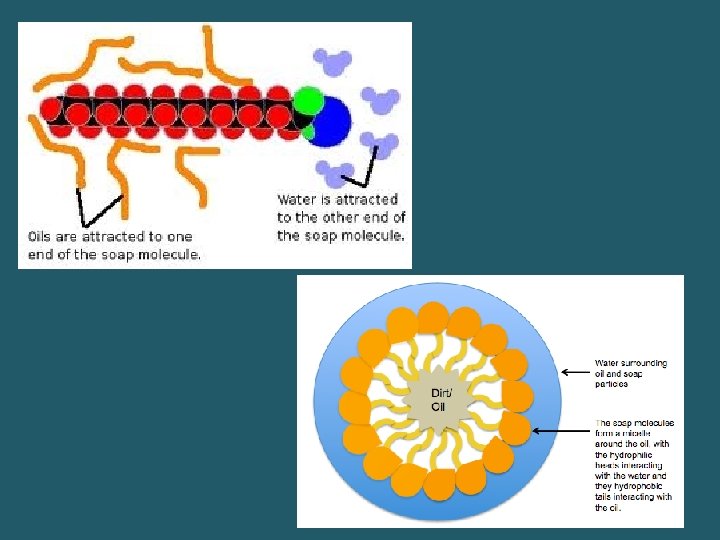
“Like Dissolves Like” • _______solutes dissolve in ______solvents • _____solutes dissolve in _____ solvents • Certain molecules can have a _______ end and _______ end (especially if they are large) – This is why _______ will dissolve grease and then be washed away in water
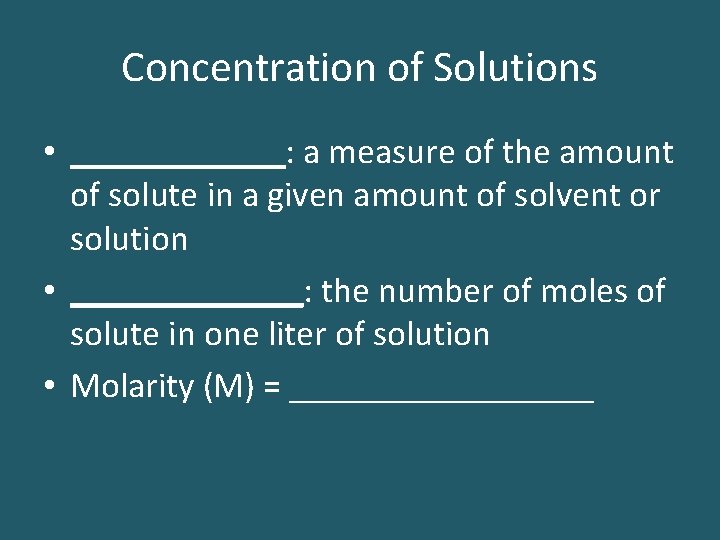
Concentration of Solutions • ______: a measure of the amount of solute in a given amount of solvent or solution • _______: the number of moles of solute in one liter of solution • Molarity (M) = _________

Molarity • One mole of Na. OH has a mass of 39. 998 g. • If this mass is dissolved in enough water to make exactly 1. 00 L of solution, the solution is a 1 M solution. • If 20. 0 g of Na. OH is dissolved in enough water to make 1. 00 L of solution, what is the molarity?

• Notice that 1 mol of solute added to 1 L of solvent does not usually make 1 M solution • Adding the solute to 1 L will cause the volume to increase

The preparation of a 0. 0128 M solution of Cu. SO 4 5 H 2 O Start by calculating the mass needed. Convert the mol to mass by multiplying by the molar mass. This mass is 3. 20 g.
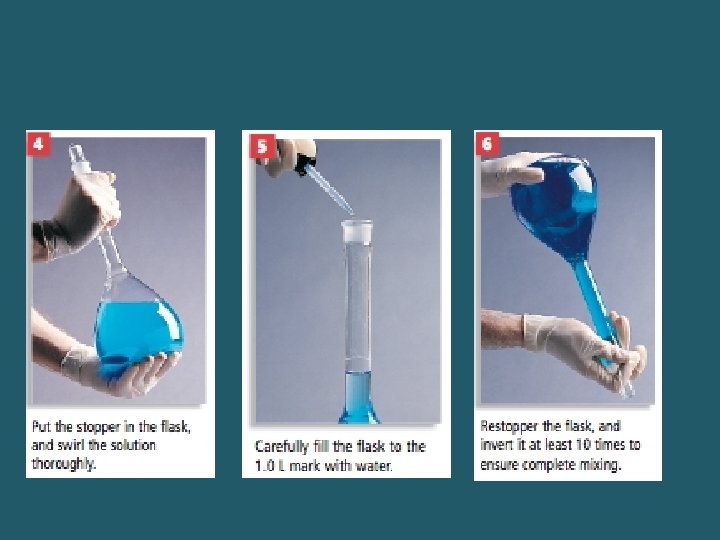

The resulting solution has. 0. 0128 mol of solute dissolved in 1. 000 L of solution, which is a 0. 0128 M concentration

Problem • You have 3. 50 L of solution that contains 90. 0 g of Na. Cl. What is the molarity of the solution?
Bellwork Molarity Problems • You have 0. 8 L of a 0. 5 M HCl solution. How many moles of HCl does this solution contain? – How many grams is this? (use 3 sig figs) • If you have 61. 9 m. L of a 2 M solution of HCl, how many moles do you have?

Dilutions • Concentrated solutions are purchased in standard molarities called ______. • You can prepare a less concentrated solution by ________ the stock solution with solvent (usually water) • The ____________does not change when you dilute

Making Dilutions • The molarity times the volume of the stock solution equals the molarity times the volume of the dilution • ___________ • Where M 1 V 1 is the molarity and volume of the stock solution and M 2 V 2 is the molarity and volume of the dilution

Problem • What volume, in m. L of 2. 00 M calcium chloride stock solution would you use to make 0. 50 L of 0. 300 M calcium chloride solution?

Problems • What volume of a 3. 00 M KI stock solution would you use to make 0. 300 L of a 1. 25 M KI solution? • How many m. L of a 5. 0 M H 2 SO 4 stock solution would you need to prepare 100. 0 m. L of a 0. 25 M H 2 SO 4? • If you dilute 20. 0 m. L of a 3. 5 M solution to make 100. 0 m. L of solution, what is the molarity of the dilute solution?
- Slides: 24