Solutions Part III IMF Energetics of Solutions Henrys



















- Slides: 33

Solutions Part III: IMF, Energetics of Solutions, Henry’s Law Much of this is basic concepts that you should review on your own. Study Slides 1 thru 14 on your own. Slides 15 thru 26 are summarized in a handout. You should review these slides on your own. Lecture begins on Slide 27. (Jespersen Chap. 13 Sec 1 – 4) Dr. C. Yau Fall 2014 1

What exactly does "solution" mean? solute + solvent = solution They can be in any physical state. Most common is solid in liquid, such as Na. Cl in water to give salt water. The only requirement is they form a HOMOGENEOUS MIXTURE, which means at the "particulate" level, particles are uniformly mixed. 2

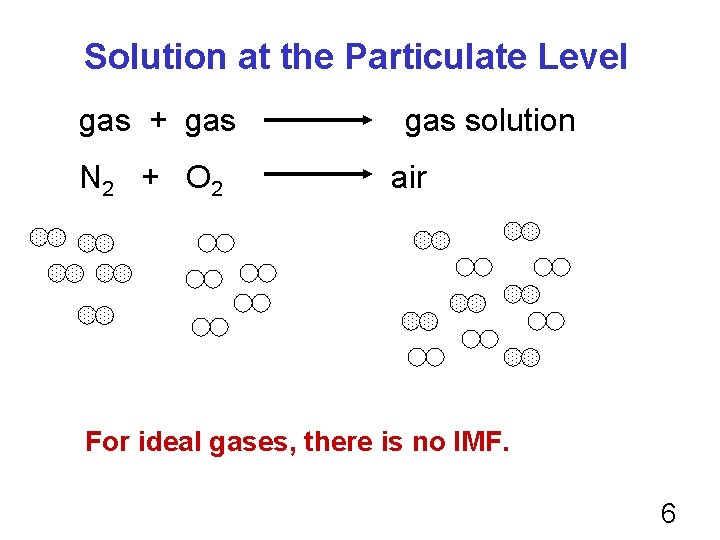
You can have a “solution” of gas in gas: e. g. Air is a homogeneous mixture of mainly N 2 and O 2. You can have a “solution of gas in liquid: e. g. O 2 dissolved in water. This is how fish “breathes. ” You can have a solution of liquid in liquid: e. g. Rubbing alcohol is a homogeneous mixture of isopropyl alcohol (liquid) in water. 3

Like Dissolves Like KNOW THESE RULES WELL! Nonpolar solutes prefer to dissolve in nonpolar solvents. Polar solutes prefer to dissolve in polar solvents. Ionic compounds are more like polar compounds than nonpolar. They prefer to dissolve in polar solvents. 4

Solution at the Particulate Level solid + solid Cu + Zn solid solution brass (an alloy) uniformly mixed at the particulate level attracted by London forces 5

Solution at the Particulate Level gas + gas N 2 + O 2 gas solution air For ideal gases, there is no IMF. 6

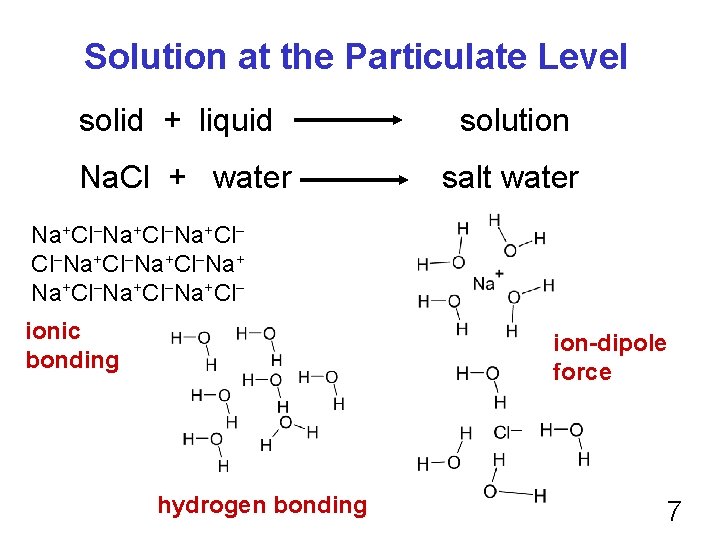
Solution at the Particulate Level solid + liquid solution Na. Cl + water salt water Na+Cl Cl Na+Cl Na+Cl ionic bonding ion-dipole force hydrogen bonding 7

8

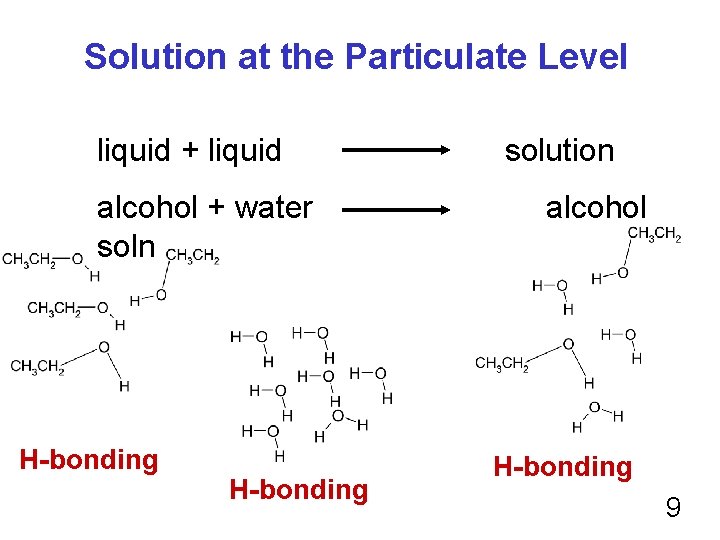
Solution at the Particulate Level liquid + liquid alcohol + water soln H-bonding solution alcohol H-bonding 9

Dissolution Of A Polar Compound In Water Dipole of the water interacts with the oppositely charged dipoles of the solid, extracting them from the crystal 10

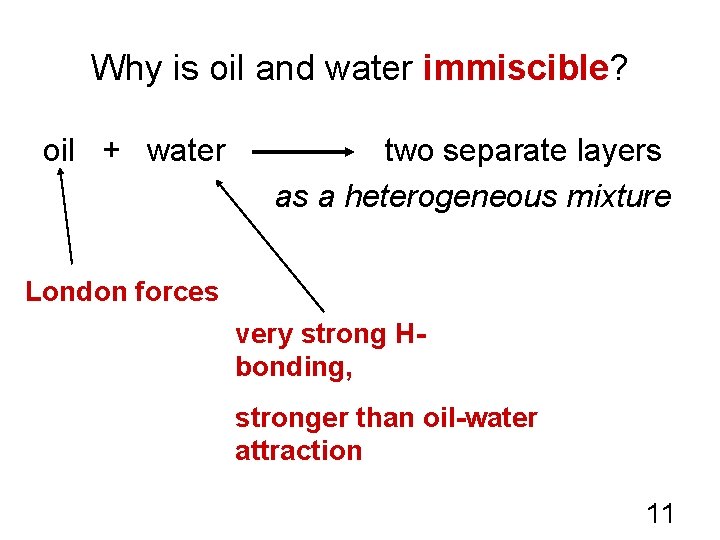
Why is oil and water immiscible? oil + water two separate layers as a heterogeneous mixture London forces very strong Hbonding, stronger than oil-water attraction 11

Miscibility of Liquids • Liquids that can dissolve in one another are miscible, while insoluble liquids are immiscible • Ethanol and water are miscible, while benzene and water are not. WHY NOT? 12

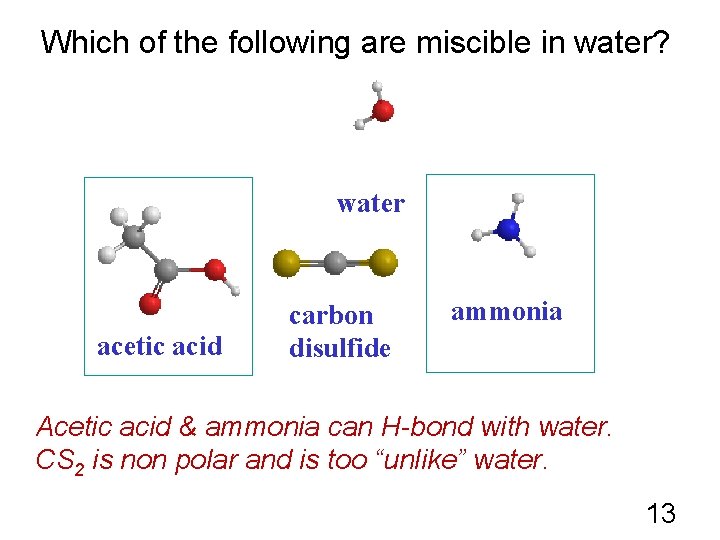
Which of the following are miscible in water? water acetic acid carbon disulfide ammonia Acetic acid & ammonia can H-bond with water. CS 2 is non polar and is too “unlike” water. 13

Which of the following are likely to be miscible with water? A. CH 3 CH 2 CH 3 B. C 6 H 6 C. CH 3 CO 2 H D. All are expected to be miscible A and B are hydrocarbons (nonpolar). C. is acetic acid and can H-bond with water. 14

Solvation is the process of solute particles becoming surrounded by solvent molecules. Hydration is the process of solute particles becoming surrounded by water molecules Hydration is solvation when the solvent is water, and the resultant solution. . . is called an "aqueous solution. " 15

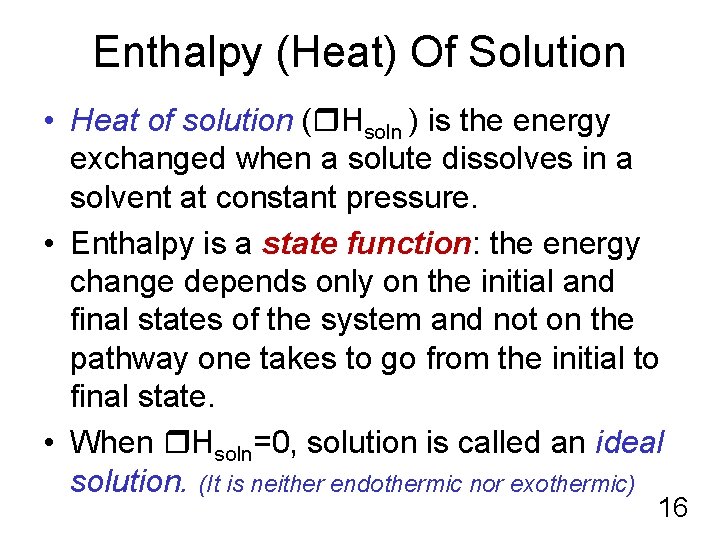
Enthalpy (Heat) Of Solution • Heat of solution ( Ηsoln ) is the energy exchanged when a solute dissolves in a solvent at constant pressure. • Enthalpy is a state function: the energy change depends only on the initial and final states of the system and not on the pathway one takes to go from the initial to final state. • When Ηsoln=0, solution is called an ideal solution. (It is neither endothermic nor exothermic) 16

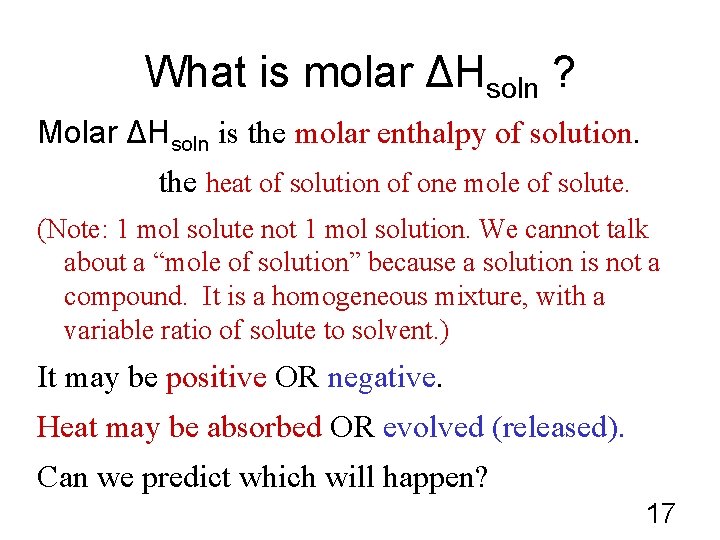
What is molar ΔHsoln ? Molar ΔHsoln is the molar enthalpy of solution. the heat of solution of one mole of solute. (Note: 1 mol solute not 1 mol solution. We cannot talk about a “mole of solution” because a solution is not a compound. It is a homogeneous mixture, with a variable ratio of solute to solvent. ) It may be positive OR negative. Heat may be absorbed OR evolved (released). Can we predict which will happen? 17

Enthalpy of Solution There are 3 steps in the process of solution: 1) Solute separates from each other (endothermic) 2) Solvent separates from each other to make room for solute (endothermic) 3) Solute fits into the space between solvent molecules (exothermic) If the net change in heat is negative, the solution process will give off heat. If the net change is positive, the soln will absorb heat (the solution feels cooler). 18

Hess’ Law REMEMBER! Just like balancing a bank account. . Need to ADD heat to separate particles of solute and solvent. Get heat back from solvation. Balance sheet? 19

Dissolution: Liquid in Liquid (Ideal) Shown here is an ideal solution where H = 0. H 0 if H 1 + H 2 H 3. (Examples on next slide. ) 20

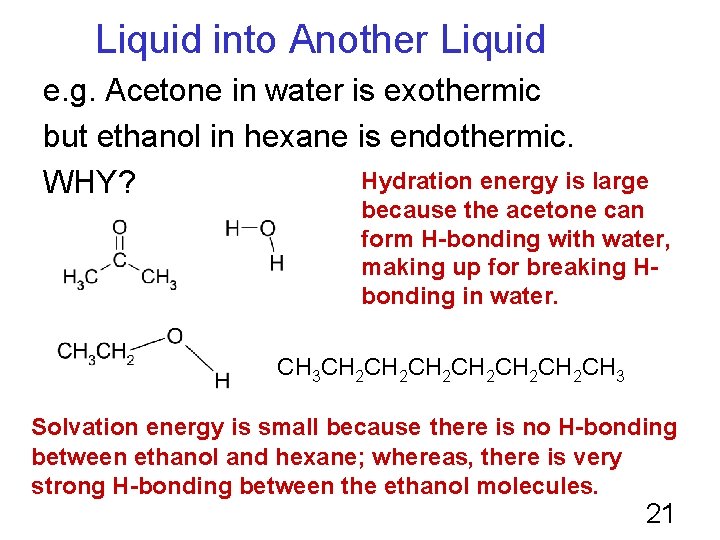
Liquid into Another Liquid e. g. Acetone in water is exothermic but ethanol in hexane is endothermic. Hydration energy is large WHY? because the acetone can form H-bonding with water, making up for breaking Hbonding in water. CH 3 CH 2 CH 2 CH 2 CH 3 Solvation energy is small because there is no H-bonding between ethanol and hexane; whereas, there is very strong H-bonding between the ethanol molecules. 21

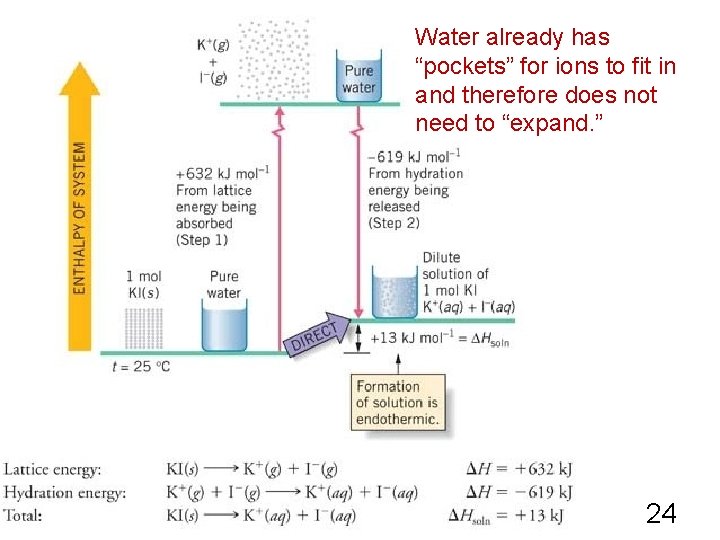
Heat of Solution of Ionic Compound in Water Step 1) Ionic compound breaks up into gaseous cations and gaseous anions This is called "lattice energy. " It is a positive value. Step 2) Gaseous ions become surrounded by water molecules. This is called "hydration energy. " It is a negative value. Overall energy change: sum of the two. 22

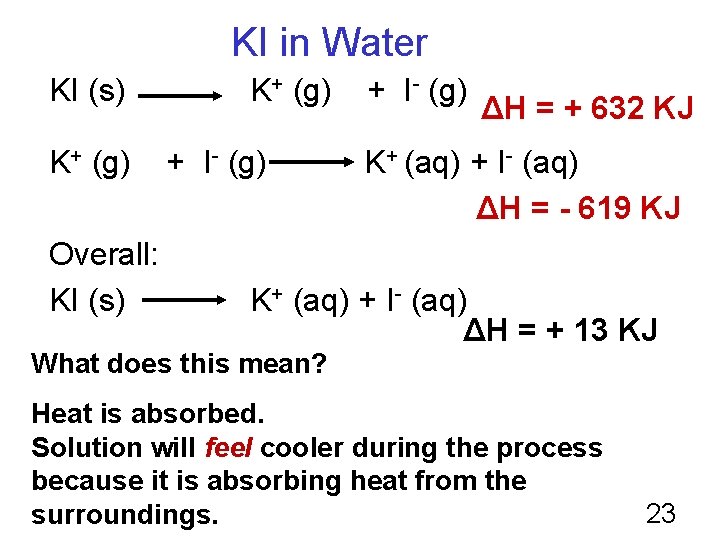
KI in Water KI (s) K+ (g) Overall: KI (s) K+ (g) + I- (g) ΔH = + 632 KJ K+ (aq) + I- (aq) ΔH = - 619 KJ K+ (aq) + I- (aq) ΔH = + 13 KJ What does this mean? Heat is absorbed. Solution will feel cooler during the process because it is absorbing heat from the surroundings. 23

Water already has “pockets” for ions to fit in and therefore does not need to “expand. ” 24

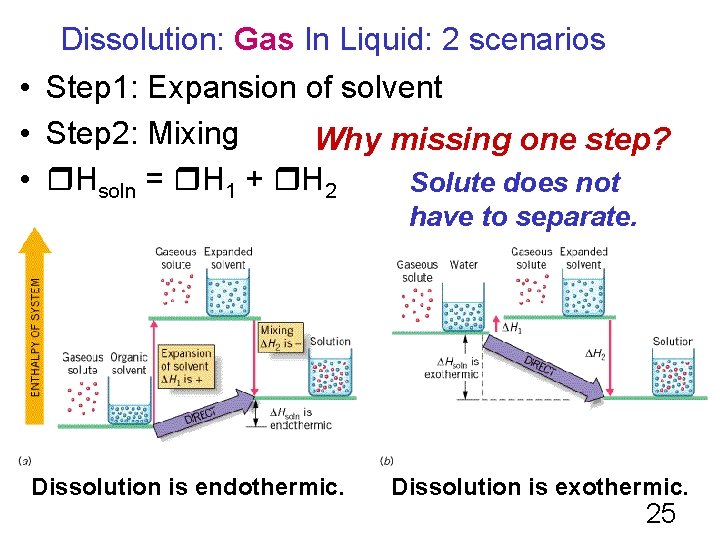
Dissolution: Gas In Liquid: 2 scenarios • Step 1: Expansion of solvent • Step 2: Mixing Why missing one step? • Ηsoln = Η 1 + Η 2 Solute does not have to separate. Dissolution is endothermic. Dissolution is exothermic. 25

Gas Solubility Heats of solution for gases in organic solvents are often endothermic. This is because it takes more energy to open a "pocket" of space in the organic solvents than the energy released when the gases go into the "pocket. " Heats of solution for gases in water are often exothermic. This is because water already has pockets to hold the molecules so it does not take any energy, but energy is released in the 26 hydration step.

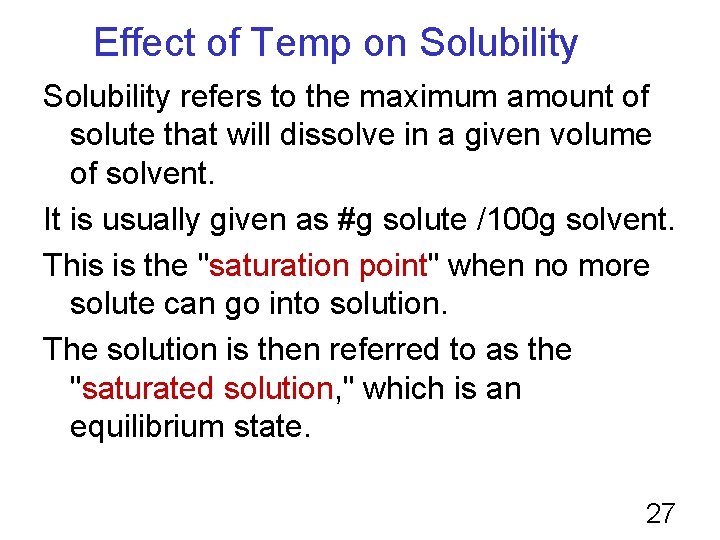
Effect of Temp on Solubility refers to the maximum amount of solute that will dissolve in a given volume of solvent. It is usually given as #g solute /100 g solvent. This is the "saturation point" when no more solute can go into solution. The solution is then referred to as the "saturated solution, " which is an equilibrium state. 27

Saturated Solution solute (undissolved) solute (dissolved) Dissolution of solids in water is usually endothermic. heat + solute(undiss) solute (dissolved) Thus, adding heat would shift the equilibrium to the right, making the substance more soluble (as is the case with sugar in water). 28

Gas in Solution In the case of a gas in an aqueous solution, it is usually exothermic. solute (undissolved) solute (dissolved) + heat If heat is added, the equilibrium will shift to the LEFT, making the gas LESS soluble. This is what you observe when you open a soda bottle in hot weather. The gas will come out much faster than in cold weather. Sodas go flat faster at rm temp than in the fridge. 29

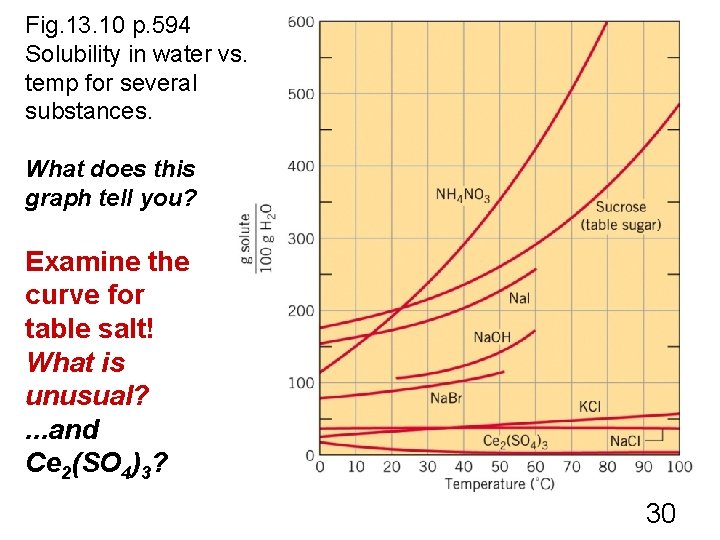
Fig. 13. 10 p. 594 Solubility in water vs. temp for several substances. What does this graph tell you? Examine the curve for table salt! What is unusual? . . . and Ce 2(SO 4)3? 30

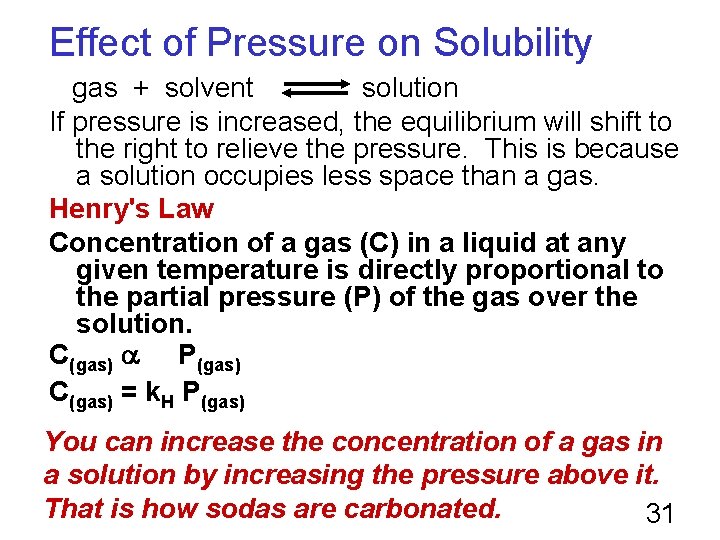
Effect of Pressure on Solubility gas + solvent solution If pressure is increased, the equilibrium will shift to the right to relieve the pressure. This is because a solution occupies less space than a gas. Henry's Law Concentration of a gas (C) in a liquid at any given temperature is directly proportional to the partial pressure (P) of the gas over the solution. C(gas) P(gas) C(gas) = k. H P(gas) You can increase the concentration of a gas in a solution by increasing the pressure above it. That is how sodas are carbonated. 31

Application of Henry's Law C(gas) = k. H P(gas) Ex. 13. 1 (p. 596) At 20 o. C the solubility of N 2 in water is 0. 0152 g L-1 when the partial pressure of nitrogen is 585. torr. What will be the solubility of N 2 in water at 20 o. C when its partial pressure is 823 torr? Ans. 0. 0214 g/L 32

Solubility of Gases in Water Some gases are more soluble than others: • Polar gases are more soluble because they form stronger attraction to the solvent. (e. g. SO 2, NH 3) • Gases that react with the solvent are more soluble because the rxn removes the product & thus the equilibrium will shift to replenish the lost product. H 2 O e. g. SO 2 (g) SO 2 (aq) H 2 SO 3 (aq) The product SO 2 (aq) is funneled off as H 2 SO 3, so the equilibrium shifts to the right to replenish the lost SO 2 (aq). 33
 Patrick henrys speech
Patrick henrys speech Henrys tires
Henrys tires Scrabble whiteboard
Scrabble whiteboard Henrys law
Henrys law Anaplerosis
Anaplerosis Glycolysis energetics
Glycolysis energetics Hmp shunt definition
Hmp shunt definition Azide electron transport chain
Azide electron transport chain Energetics power tower 180
Energetics power tower 180 Gluconeogenesis from lactate
Gluconeogenesis from lactate Building energetics
Building energetics Ib energetics
Ib energetics Energetics janesville
Energetics janesville Chemsheets a2 1048 answers
Chemsheets a2 1048 answers Hamlet act iii scene ii
Hamlet act iii scene ii Roles of imf
Roles of imf Imf adalah
Imf adalah Imf chem
Imf chem Difference between imf and world bank
Difference between imf and world bank Materi lembaga keuangan internasional
Materi lembaga keuangan internasional Imf voting power
Imf voting power India imf loan history
India imf loan history Imf practice
Imf practice Wto objectives
Wto objectives Imf chemistry
Imf chemistry Imf system
Imf system Debt imf
Debt imf Lambang imf
Lambang imf Imf chemistry
Imf chemistry Imf
Imf Imf
Imf Imf
Imf Imf gdp per capita
Imf gdp per capita Factors affecting vapor pressure
Factors affecting vapor pressure