Solutions Part I Colligative Properties loosely from Jespersen














- Slides: 34

Solutions Part I: Colligative Properties (loosely from Jespersen Chap. 13 Sec 6 & 7 Dr. C. Yau Fall 2013

What are Solutions? Quick reminder: Solutions are homogeneous mixtures consisting of. . . generally one solute dissolved in a solvent. (More than one solute is possible. ) 2

Colligative Properties The term "colligative" comes from a Greek word meaning "dependent on the number. " Colligative properties refer to the properties of the solvent that depends on the number of particles (of the solute) and not the nature of the particles. However, with the stipulation that. . . These solutes must be nonvolatile (and therefore does not contribute to the vapor pressure of the solvent). 3

Colligative Properties Since the properties are NOT dependent on the "nature" of the solute, this means the IMF of the solute plays no part in this. We will be studying how the concentration of the solute affects the properties of the solvent. 4

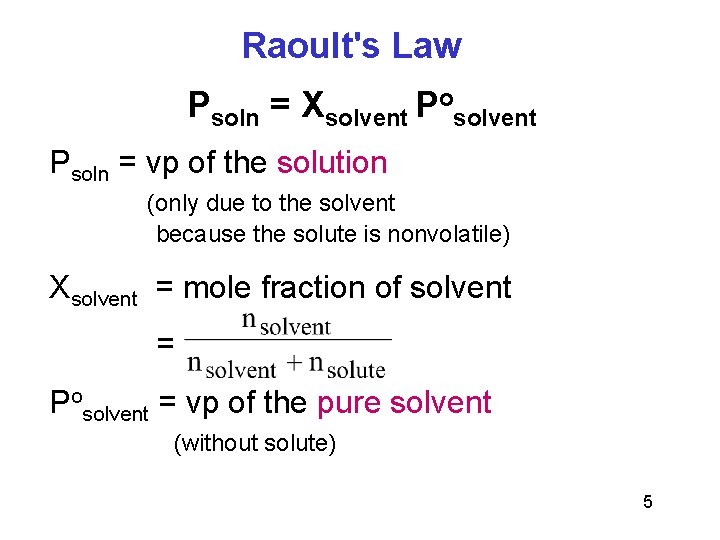
Raoult's Law Psoln = Xsolvent Posolvent Psoln = vp of the solution (only due to the solvent because the solute is nonvolatile) Xsolvent = mole fraction of solvent = Posolvent = vp of the pure solvent (without solute) 5

Raoult's Law Psoln = Xsolvent Posolvent Vp of solution is a fraction of the vp of the pure solvent. This means that the solute has lowered the vp of the solvent. Why? 6

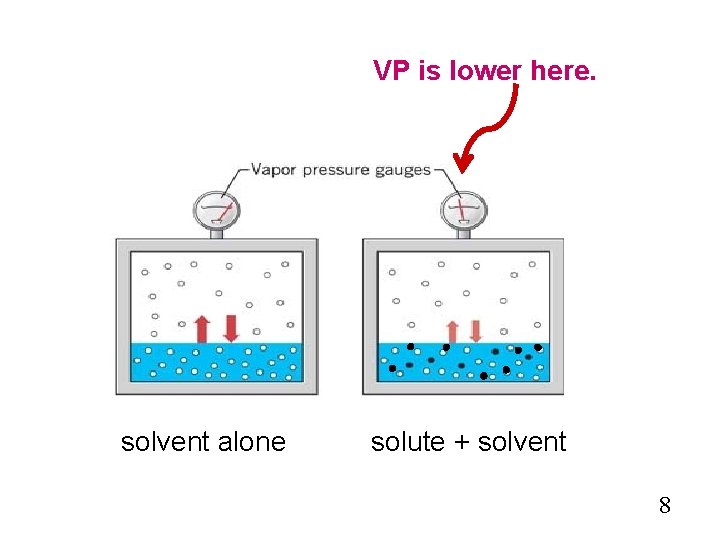
VP Lowering One way to explain it is to consider the entropy of the system (which will be covered in a later chapter). Another way is to consider the solute particles at the surface of the solution. Their presence decreases the number of solvent particles on the surface, thus decreasing the chances of them breaking off into the vapor phase. 7

VP is lower here. solvent alone solute + solvent 8

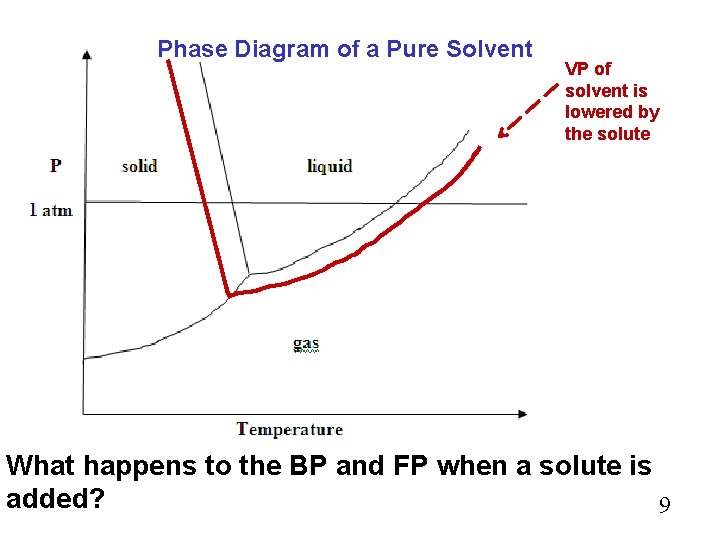
Phase Diagram of a Pure Solvent VP of solvent is lowered by the solute What happens to the BP and FP when a solute is added? 9

Effect of Solute on MP and BP Again we are considering only solutes that are nonvolatile… The BP is raised and the FP is lowered by the presence of the solute. These are referred to as… BP elevation and FP depression. 10

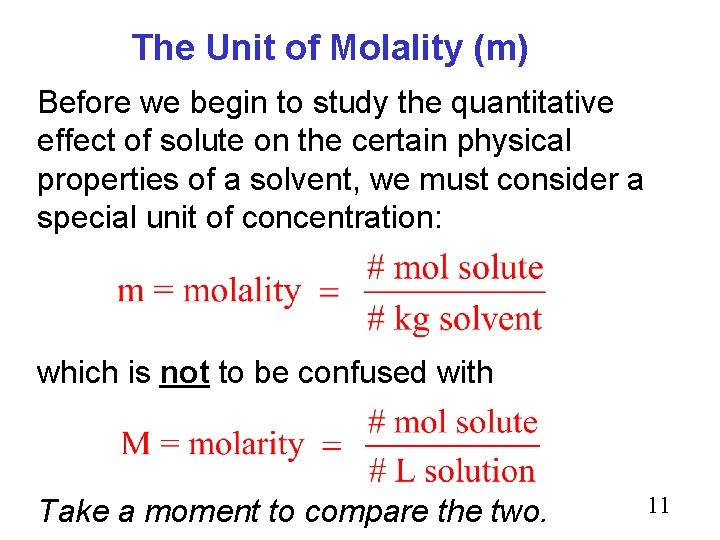
The Unit of Molality (m) Before we begin to study the quantitative effect of solute on the certain physical properties of a solvent, we must consider a special unit of concentration: which is not to be confused with Take a moment to compare the two. 11

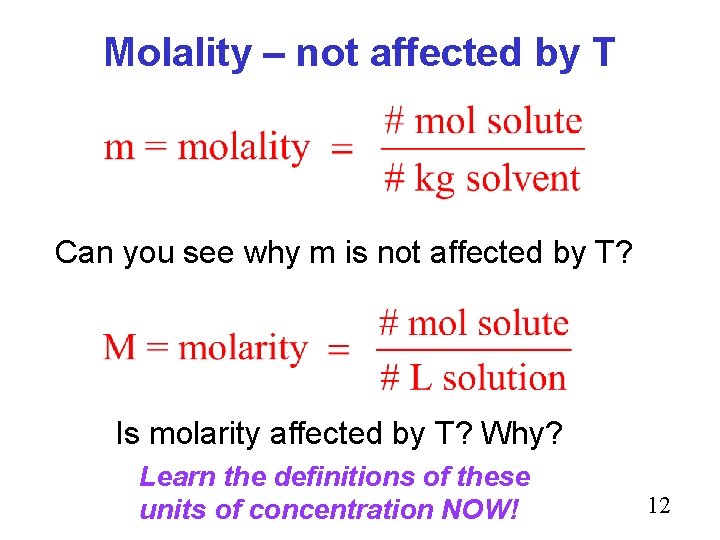
Molality – not affected by T Can you see why m is not affected by T? Is molarity affected by T? Why? Learn the definitions of these units of concentration NOW! 12

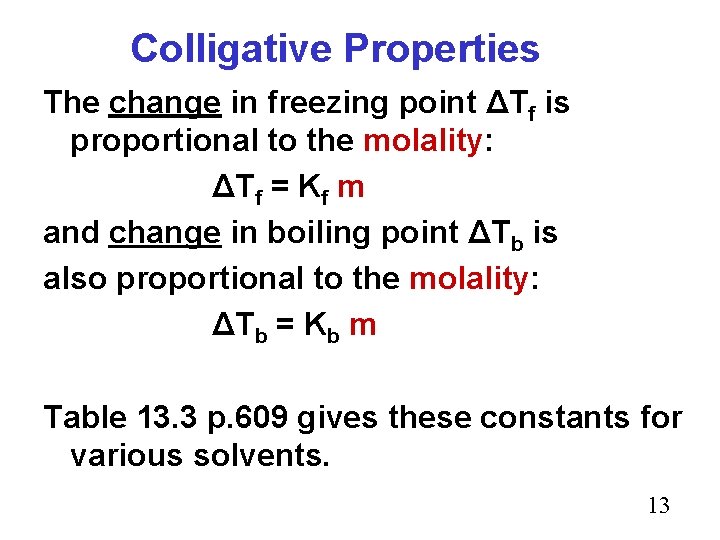
Colligative Properties The change in freezing point ΔTf is proportional to the molality: ΔTf = Kf m and change in boiling point ΔTb is also proportional to the molality: ΔTb = Kb m Table 13. 3 p. 609 gives these constants for various solvents. 13

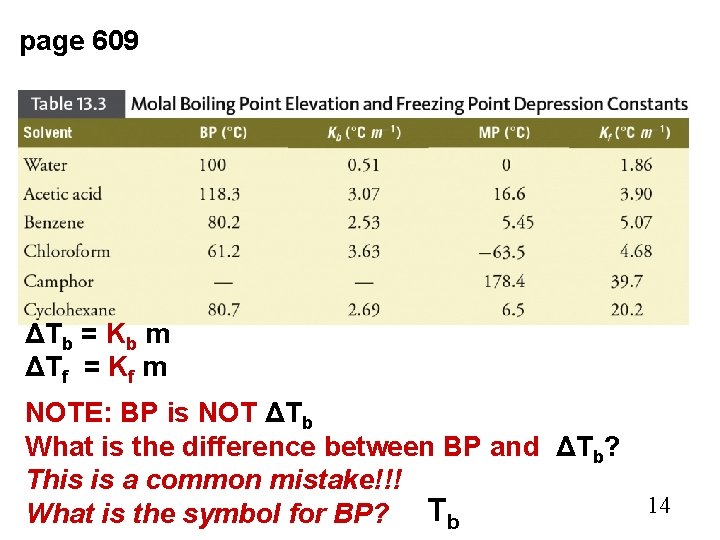
page 609 ΔTb = Kb m ΔTf = Kf m NOTE: BP is NOT ΔTb What is the difference between BP and ΔTb? This is a common mistake!!! 14 What is the symbol for BP? Tb

Determination of fp of solution Example 13. 8 p. 609 Estimate the fp of a solution made from 10. 0 g of urea, CO(NH 2)2, with MM 60. 06 g mol-1, and 125 g of water. Table 13. 3 tells us Kf for water = 1. 86 o. C m-1 (or o. C/m) Do Practice Exercise 17 & 18 p. 610. 15

Determination of MM from Colligative Properties Example 13. 9 p. 611 A solution made by dissolving 5. 65 g of an unknown molecular compound in 110. 0 g of benzene froze at 4. 39 o. C. What is the molar mass of the solute? Table 13. 3 tells us Kf for benzene = 5. 07 o. C/m Fp for benzene = 5. 45 o. C Do Practice Exercise 19 & 20 p. 612. 16

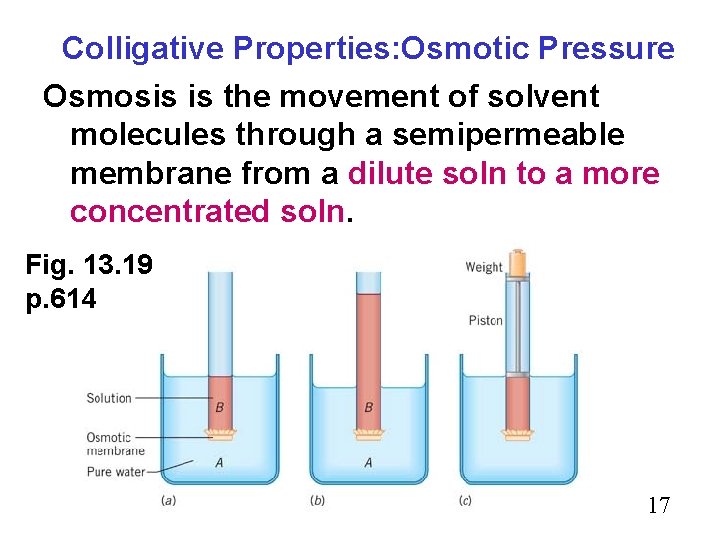
Colligative Properties: Osmotic Pressure Osmosis is the movement of solvent molecules through a semipermeable membrane from a dilute soln to a more concentrated soln. Fig. 13. 19 p. 614 17

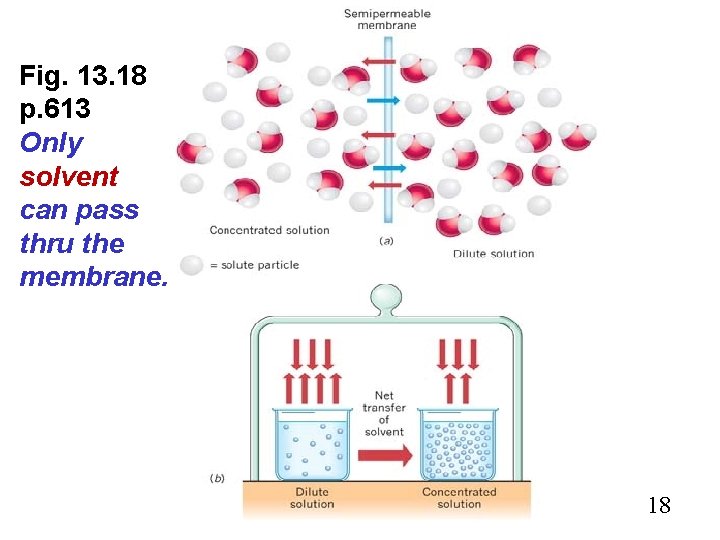
Fig. 13. 18 p. 613 Only solvent can pass thru the membrane. 18

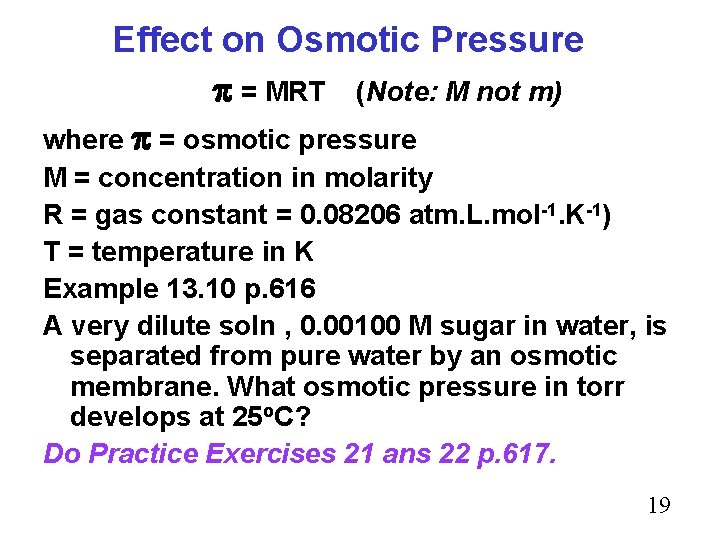
Effect on Osmotic Pressure = MRT (Note: M not m) where = osmotic pressure M = concentration in molarity R = gas constant = 0. 08206 atm. L. mol-1. K-1) T = temperature in K Example 13. 10 p. 616 A very dilute soln , 0. 00100 M sugar in water, is separated from pure water by an osmotic membrane. What osmotic pressure in torr develops at 25 o. C? Do Practice Exercises 21 ans 22 p. 617. 19

Determination of MM from Osmotic Pressure Example 13. 11 p. 617 An aqueous solution with a volume of 100. m. L and containing 0. 122 g of an unknown molecular compound has an osmotic pressure of 16. 0 torr at 21. 0 o. C. What is the molar mass of the solute? Do Practice Exercises 23 & 24 p. 618. 20

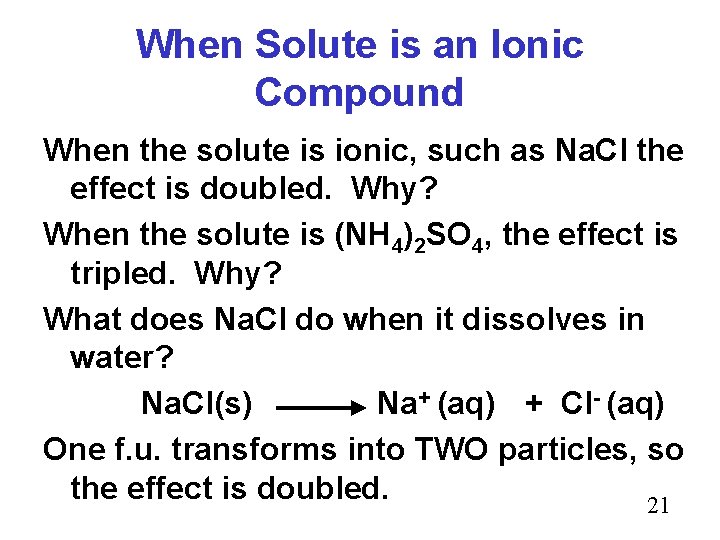
When Solute is an Ionic Compound When the solute is ionic, such as Na. Cl the effect is doubled. Why? When the solute is (NH 4)2 SO 4, the effect is tripled. Why? What does Na. Cl do when it dissolves in water? Na. Cl(s) Na+ (aq) + Cl- (aq) One f. u. transforms into TWO particles, so the effect is doubled. 21

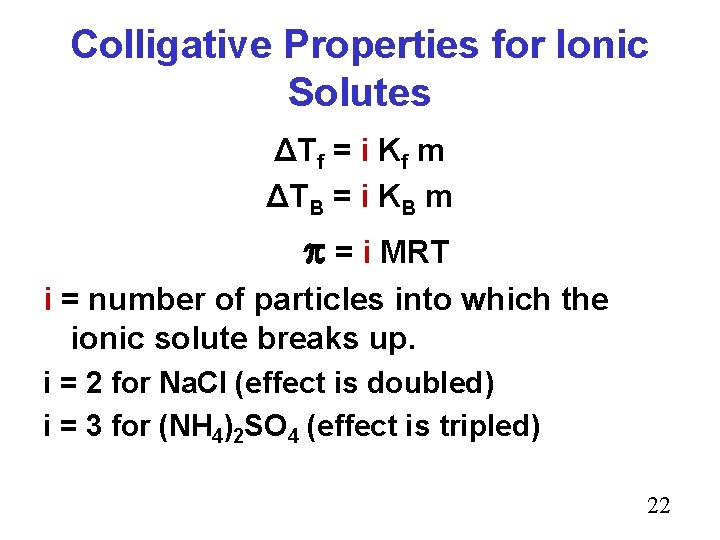
Colligative Properties for Ionic Solutes ΔTf = i Kf m ΔTB = i KB m = i MRT i = number of particles into which the ionic solute breaks up. i = 2 for Na. Cl (effect is doubled) i = 3 for (NH 4)2 SO 4 (effect is tripled) 22

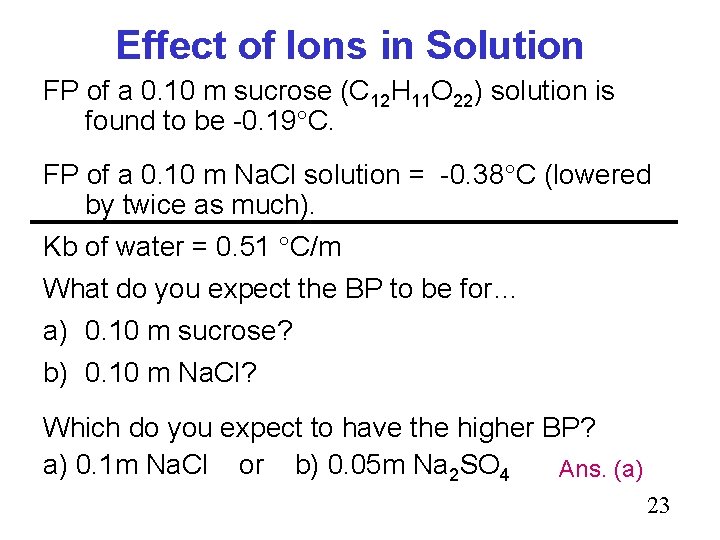
Effect of Ions in Solution FP of a 0. 10 m sucrose (C 12 H 11 O 22) solution is found to be -0. 19 C. FP of a 0. 10 m Na. Cl solution = -0. 38 C (lowered by twice as much). Kb of water = 0. 51 C/m What do you expect the BP to be for… a) 0. 10 m sucrose? b) 0. 10 m Na. Cl? Which do you expect to have the higher BP? a) 0. 1 m Na. Cl or b) 0. 05 m Na 2 SO 4 Ans. (a) 23

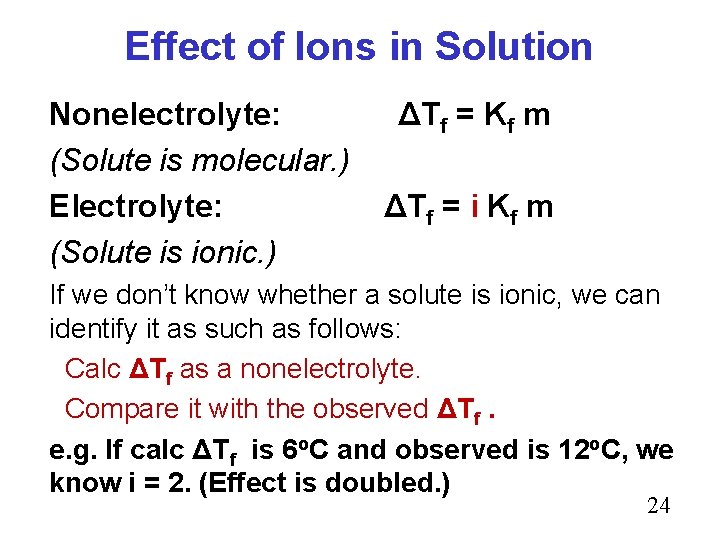
Effect of Ions in Solution Nonelectrolyte: (Solute is molecular. ) Electrolyte: (Solute is ionic. ) ΔTf = Kf m ΔTf = i Kf m If we don’t know whether a solute is ionic, we can identify it as such as follows: Calc ΔTf as a nonelectrolyte. Compare it with the observed ΔTf. e. g. If calc ΔTf is 6 o. C and observed is 12 o. C, we know i = 2. (Effect is doubled. ) 24

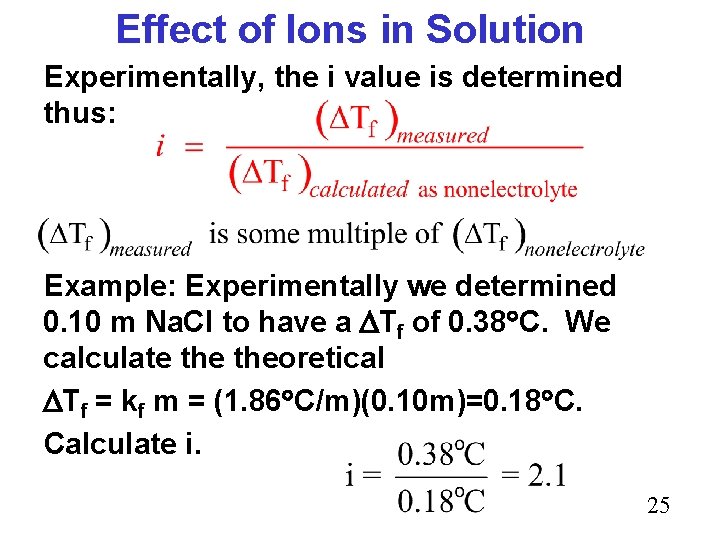
Effect of Ions in Solution Experimentally, the i value is determined thus: Example: Experimentally we determined 0. 10 m Na. Cl to have a Tf of 0. 38 C. We calculate theoretical Tf = kf m = (1. 86 C/m)(0. 10 m)=0. 18 C. Calculate i. 25

Effect of concentration on i • i value is affected by concentration! • WHY? • When the concentration increases, the ions are closer together and the attraction between them increases. • They begin to act less freely as dissociated ions (less than 100% dissociated). 26

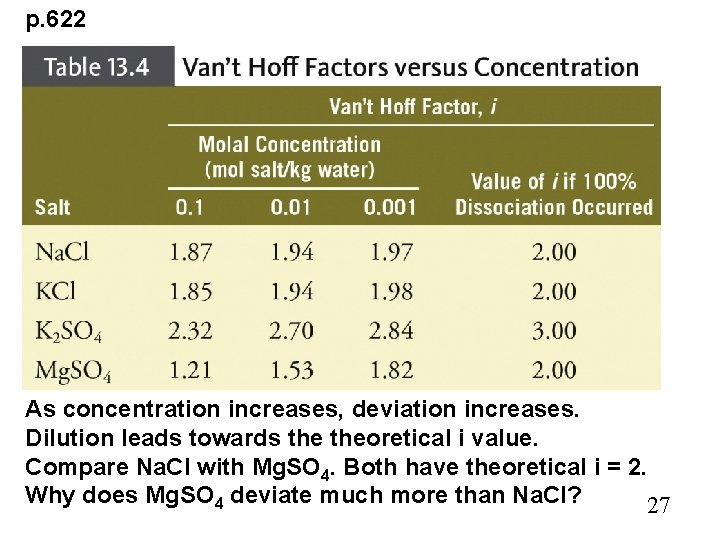
p. 622 As concentration increases, deviation increases. Dilution leads towards theoretical i value. Compare Na. Cl with Mg. SO 4. Both have theoretical i = 2. Why does Mg. SO 4 deviate much more than Na. Cl? 27

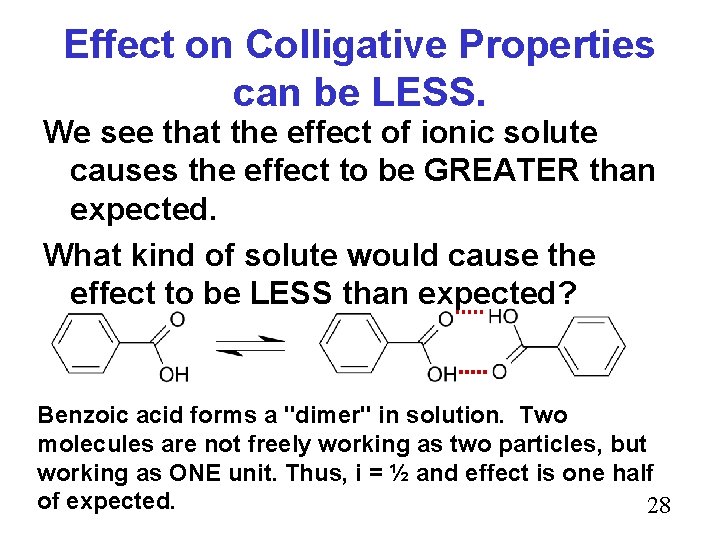
Effect on Colligative Properties can be LESS. We see that the effect of ionic solute causes the effect to be GREATER than expected. What kind of solute would cause the effect to be LESS than expected? Benzoic acid forms a "dimer" in solution. Two molecules are not freely working as two particles, but working as ONE unit. Thus, i = ½ and effect is one half of expected. 28

Vapor Pressure Lowering We had previously begun with the explanations to why the vapor pressure of a solvent is lowered when a solute is added. Let us now return to “vapor pressure lowering” and examine the implications. 29

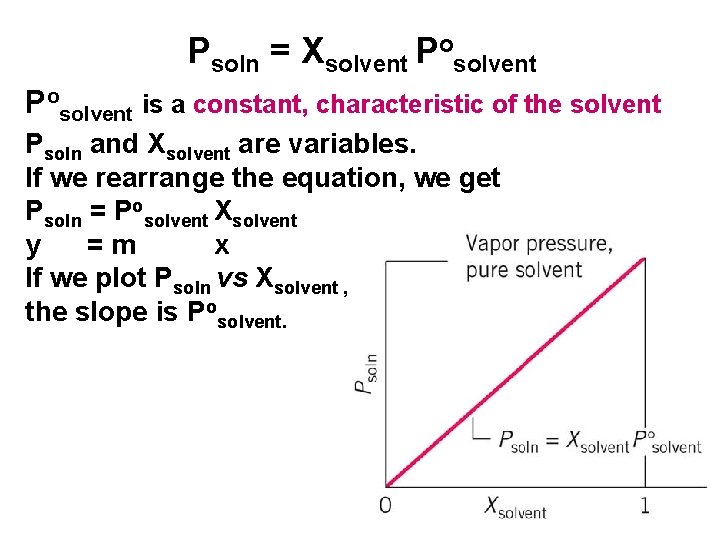
Psoln = Xsolvent Posolvent is a constant, characteristic of the solvent Psoln and Xsolvent are variables. If we rearrange the equation, we get Psoln = Posolvent Xsolvent y =m x If we plot Psoln vs Xsolvent , the slope is Posolvent. 30

Example 13. 6 p. 604 Carbon tetrachloride has a vp of 155 torr at 20 o. C. This solvent can dissolve candle wax, which is essentially nonvolatile. Although candle wax is a mixture, we can take its molecular formula to be C 22 H 46 (MM 311 g mol-1). What is the vapor pressure at 20 o. C of a solution prepared by dissolving 10. 0 g of wax in 40. 0 g of CCl 4 (MM 154 g mol-1). Do Practice Exercises 13 & 14 p. 605 31

By how much is the vp lowered? P = change in vp due to solutes =? P = Xsolute P°solv How do we get this equation? (See lecture notes. This was derived in class. It is also in your textbook, more or less. ) 32

VP of soln if solute is volatile Let us now consider the case when the solute is VOLATILE. Applying Dalton's Law of Partial Pressures to a mixture of gases A + B, PTotal = PA + PB where PA is partial pressure of A = XAPo. A and PB is partial pressure of B = XBPo. B PTotal = XAPo. A + XBPo. B Raoult's equation is simply dropping off one term (such as XBPo. B because the solute is nonvolatile). 33 33

Raoult's Law One last point about Raoult's Law…. It is for the ideal solutions. BUT, even deviations from the law gives us information on the IMF of the solute to the solvent and IMF of the solvent molecules to solvent molecules. If the solute-solvent IMF is strong, vp of solution would be lowered more than expected. If the solvent-solvent IMF is strong, vp of the solution would not be lowered as much as expected (adding solute disrupts the IMF of the solvent and allows more solvent to vaporize). 3434