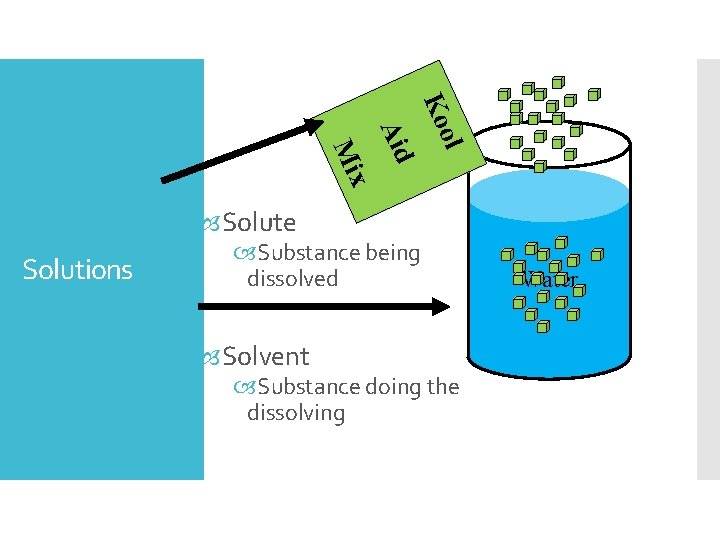
Solutions ol Ko Aid x Mi Solute Solutions





- Slides: 21

Solutions

ol Ko Aid x Mi Solute Solutions Substance being dissolved Solvent Substance doing the dissolving Water

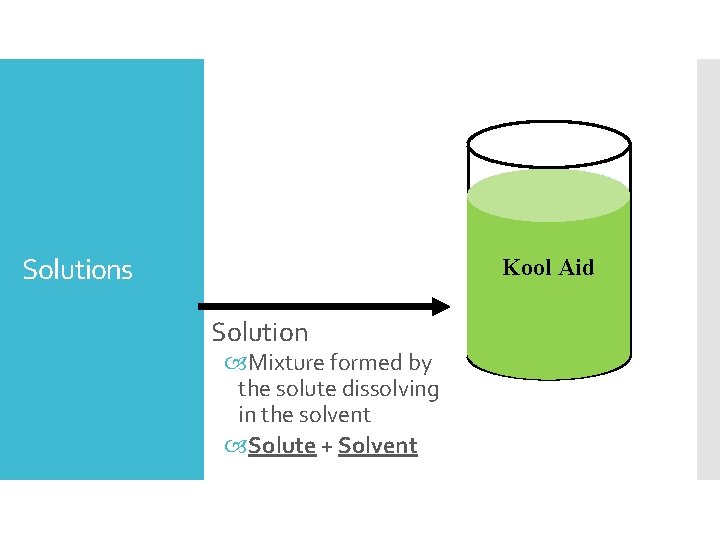
Solutions Kool Aid Solution Mixture formed by the solute dissolving in the solvent Solute + Solvent

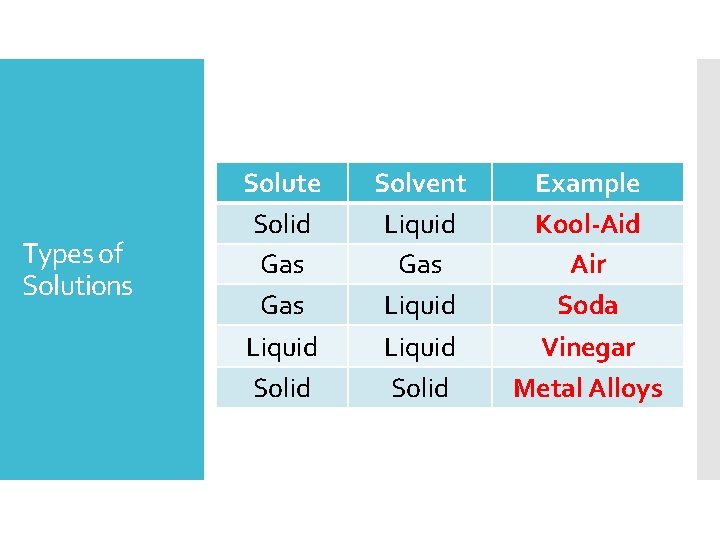
Types of Solutions Solute Solid Gas Liquid Solvent Liquid Gas Liquid Solid Example Kool-Aid Air Soda Vinegar Metal Alloys

Factors Affecting Solubility

Factors Affecting Dissolving Surface Area – size of the solute particles Agitation – dispersing solute particles Temperature – speed of the particles Pressure – stress on equilibrium of vapor and liquid states of gas Polarity – polar or nonpolar Already dissolved solute – purity of solvent

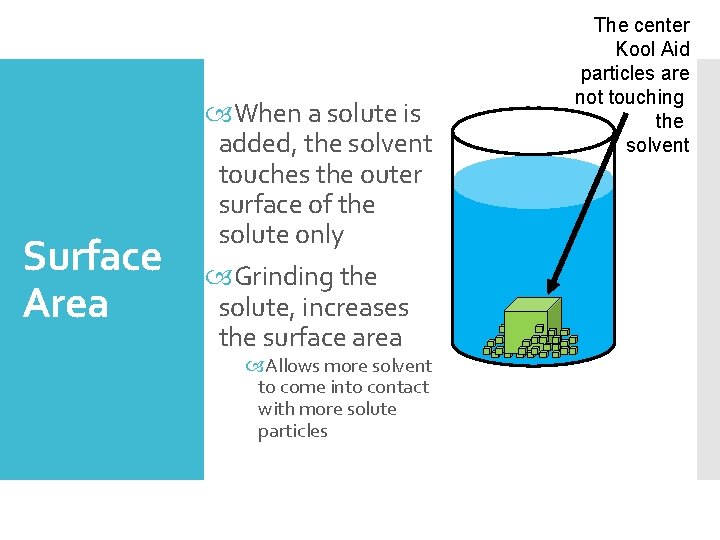
Surface Area When a solute is added, the solvent touches the outer surface of the solute only Grinding the solute, increases the surface area Allows more solvent to come into contact with more solute particles The center Kool Aid particles are not touching the solvent

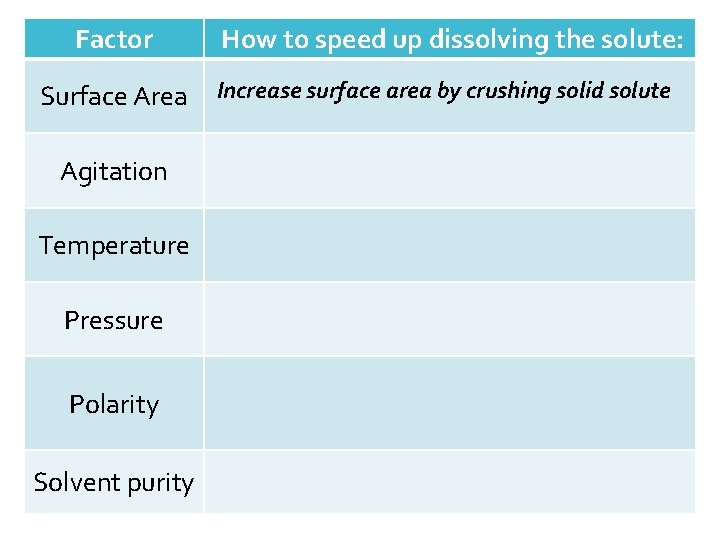
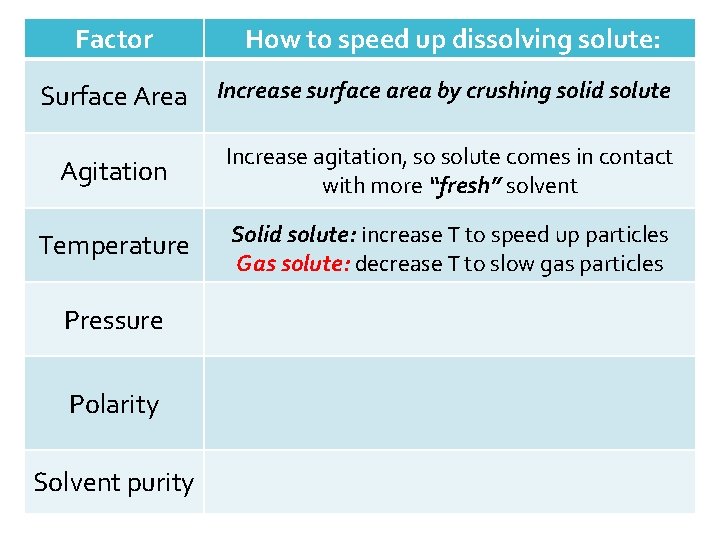
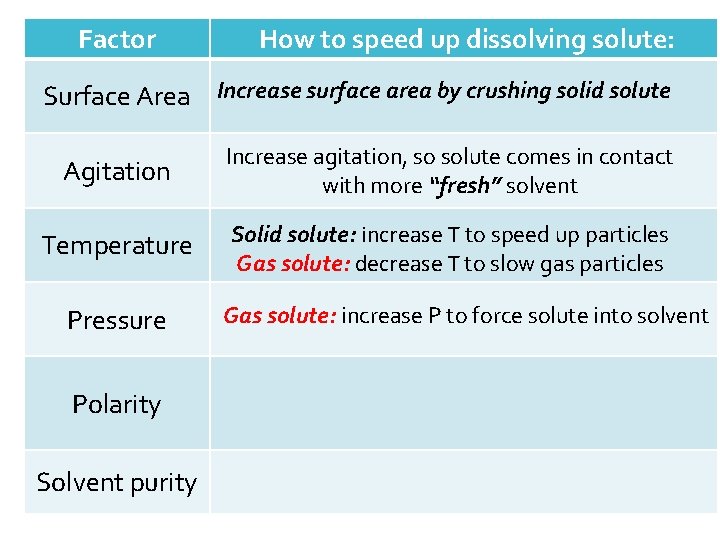
Factor Surface Area Agitation Temperature Pressure Polarity Solvent purity How to speed up dissolving the solute: Increase surface area by crushing solid solute

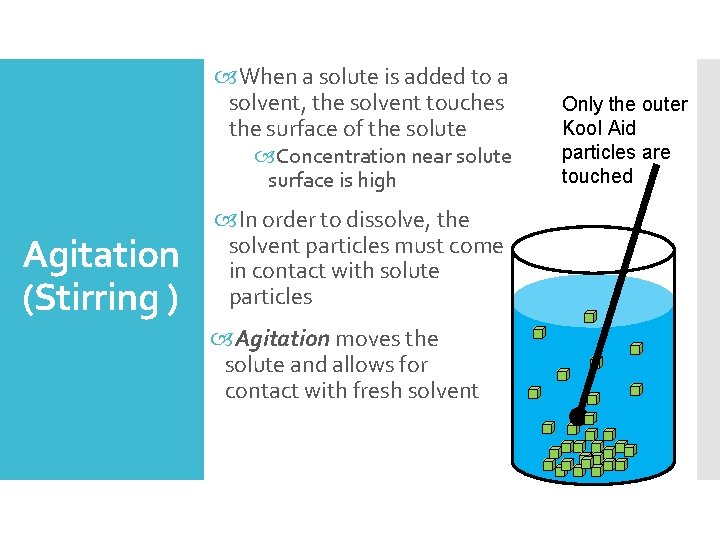
When a solute is added to a solvent, the solvent touches the surface of the solute Concentration near solute surface is high Agitation (Stirring ) In order to dissolve, the solvent particles must come in contact with solute particles Agitation moves the solute and allows for contact with fresh solvent Only the outer Kool Aid particles are touched

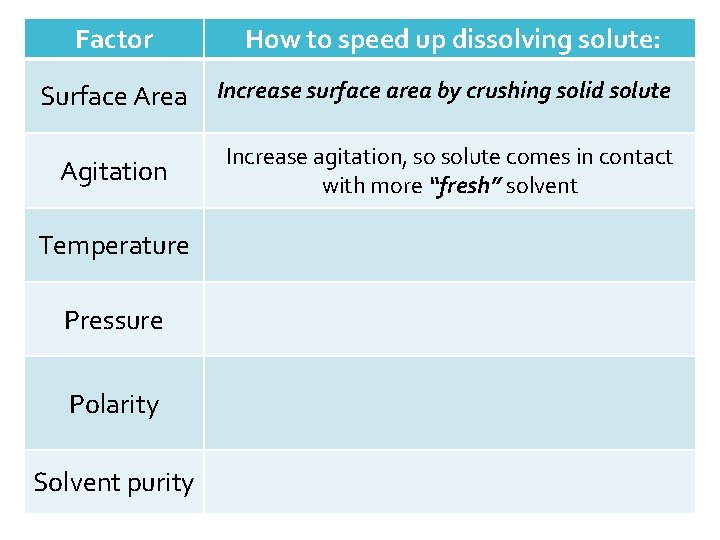
Factor How to speed up dissolving solute: Surface Area Increase surface area by crushing solid solute Agitation Increase agitation, so solute comes in contact with more “fresh” solvent Temperature Pressure Polarity Solvent purity

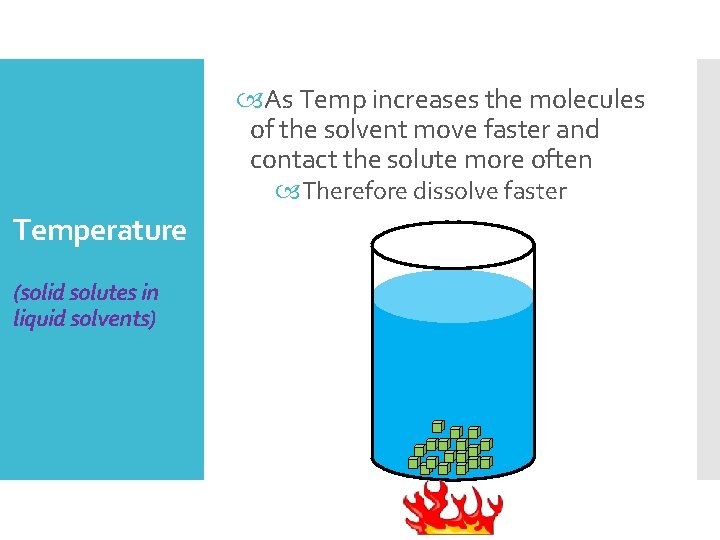
As Temp increases the molecules of the solvent move faster and contact the solute more often Therefore dissolve faster Temperature (solid solutes in liquid solvents)

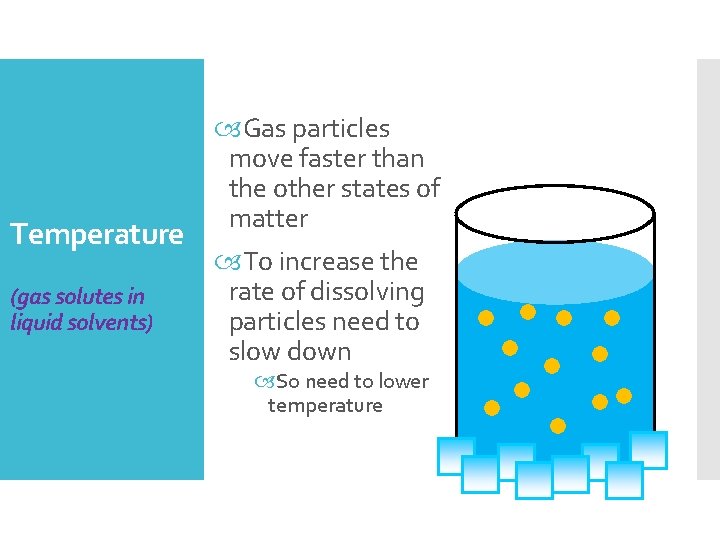
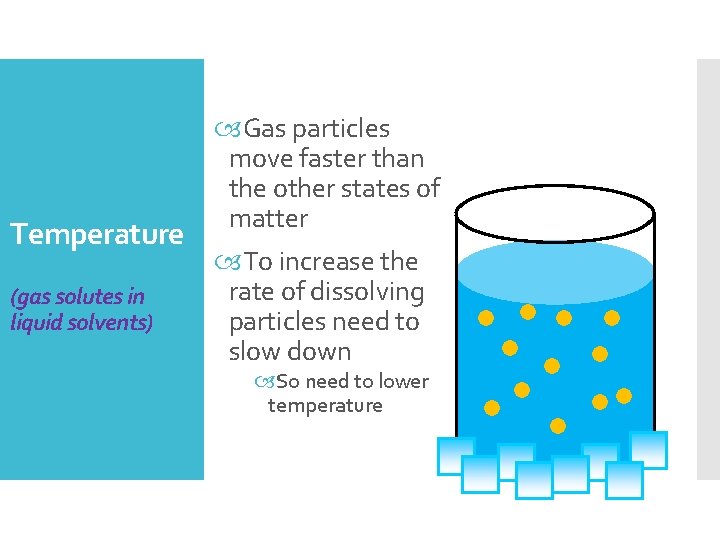
Temperature (gas solutes in liquid solvents) Gas particles move faster than the other states of matter To increase the rate of dissolving particles need to slow down So need to lower temperature

Temperature (gas solutes in liquid solvents) Gas particles move faster than the other states of matter To increase the rate of dissolving particles need to slow down So need to lower temperature

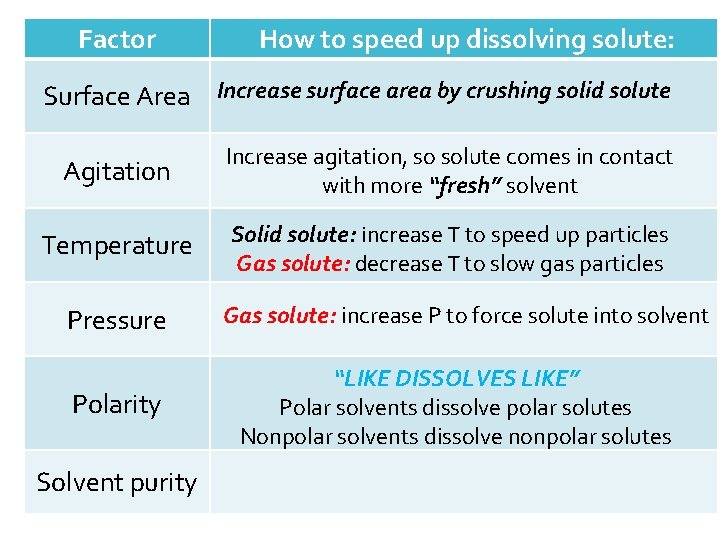
Factor How to speed up dissolving solute: Surface Area Increase surface area by crushing solid solute Agitation Increase agitation, so solute comes in contact with more “fresh” solvent Temperature Solid solute: increase T to speed up particles Gas solute: decrease T to slow gas particles Pressure Polarity Solvent purity

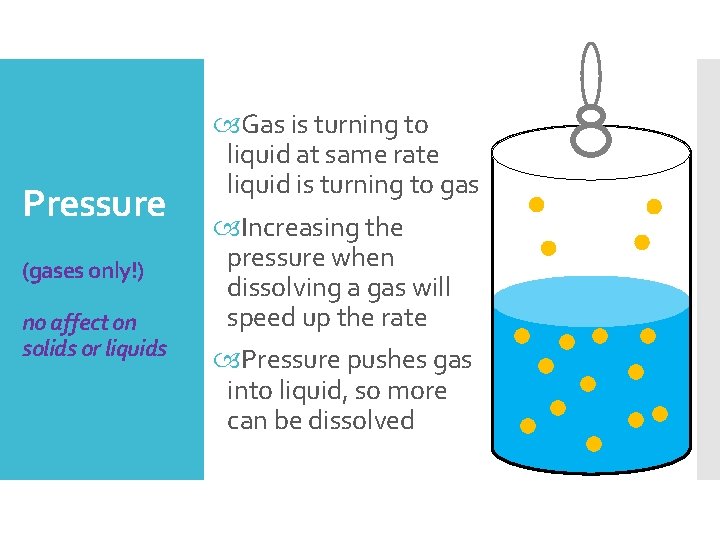
Pressure (gases only!) no affect on solids or liquids Gas is turning to liquid at same rate liquid is turning to gas Increasing the pressure when dissolving a gas will speed up the rate Pressure pushes gas into liquid, so more can be dissolved

Factor How to speed up dissolving solute: Surface Area Increase surface area by crushing solid solute Agitation Increase agitation, so solute comes in contact with more “fresh” solvent Temperature Solid solute: increase T to speed up particles Gas solute: decrease T to slow gas particles Pressure Polarity Solvent purity Gas solute: increase P to force solute into solvent

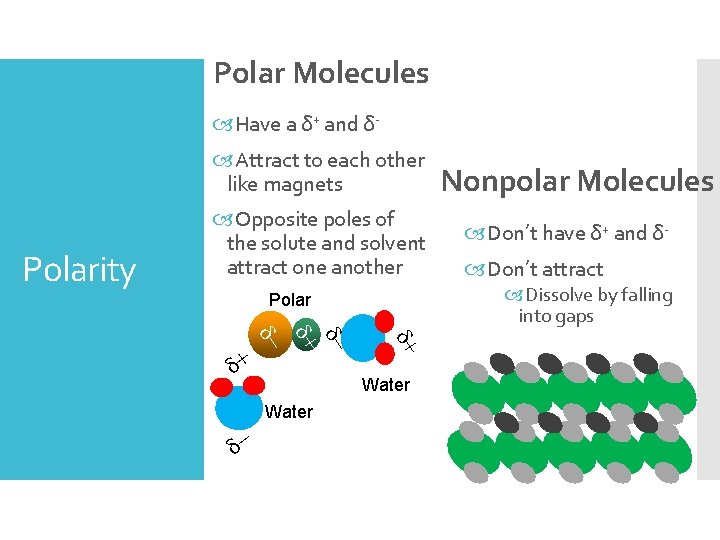
Polar Molecules Have a δ+ and δ Attract to each other like magnets Polarity Opposite poles of the solute and solvent attract one another d- d+ d- Water Don’t have δ+ and δ Don’t attract Dissolve by falling into gaps Polar d+ Nonpolar Molecules

Like (solvents) dissolve like (solutes) Basically: “Like Dissolves Like” Polar solvent dissolves polar solute Nonpolar solvents dissolve nonpolar solutes Water is a common and universal solvent Many solutions use water (b/c VERY polar)

Factor How to speed up dissolving solute: Surface Area Increase surface area by crushing solid solute Agitation Increase agitation, so solute comes in contact with more “fresh” solvent Temperature Solid solute: increase T to speed up particles Gas solute: decrease T to slow gas particles Pressure Polarity Solvent purity Gas solute: increase P to force solute into solvent “LIKE DISSOLVES LIKE” Polar solvents dissolve polar solutes Nonpolar solvents dissolve nonpolar solutes

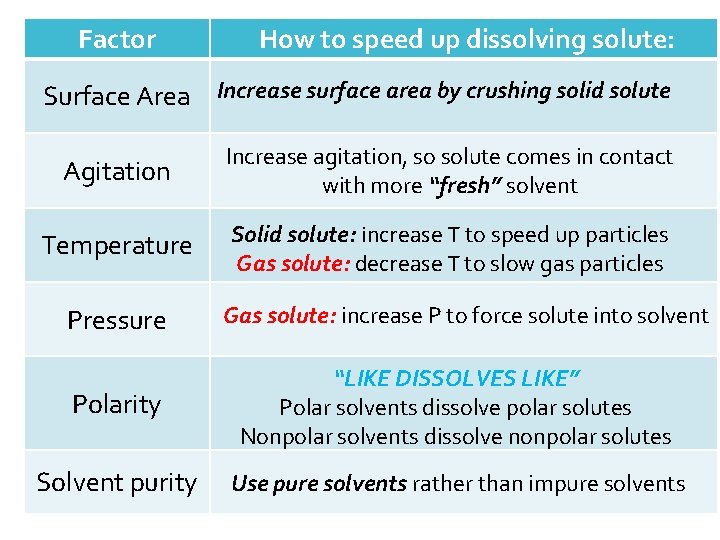
Already dissolved solute (impure solvent) Pure solvent dissolves a solute faster than an impure solvent This is because eventually you reach a point of saturation when no more solute will dissolve

Factor How to speed up dissolving solute: Surface Area Increase surface area by crushing solid solute Agitation Increase agitation, so solute comes in contact with more “fresh” solvent Temperature Solid solute: increase T to speed up particles Gas solute: decrease T to slow gas particles Pressure Gas solute: increase P to force solute into solvent Polarity “LIKE DISSOLVES LIKE” Polar solvents dissolve polar solutes Nonpolar solvents dissolve nonpolar solutes Solvent purity Use pure solvents rather than impure solvents