Solutions Ions and Concentration Calculations Ionic Salt in












- Slides: 14

Solutions, Ions, and Concentration Calculations

Ionic Salt in Water • Ionic solids will dissociate when placed in water – They go from their solid phase to ions (aqueous) – Na. Cl → Na+ + Cl- • Water molecules can attach themselves to a surface ion due to dipole-dipole forces and remove it from the crystal lattice – The positive and negative ions are attracted • When surrounded by attached water molecules, ions are said to be hydrated (hydrate molecules)

Ionic Salt in Water • A general term for this interaction between solute and solvent particles is called “solvation” • A typical dissociate equation looks like – Al. Cl 3 → Al+ + 3 Cl– 2 Na 2 O → 4 Na+ + O 2 - • Solvation can also happen in polar molecules (as opposed to ions) in water – e. g. Methanol/H 2 O

Ionic Salt in Water • Solvation does not occur between polar and non-polar substances • If both solvent and solute are non-polar, solvation may occur (through weaker Van der Waal’s forces which are momentary dipoles caused by the nucleus of one atom briefly attracting the electrons of another atom) – E. g. Benzene will dissolve moth balls (pdichlorobenzene)

Liquids dissolving in Liquids • Yes, we refer to how liquids mix with one another as being miscible (soluble) or being immiscible (insoluble) • Polar liquids will be miscible with another polar liquid – Water and alcohol • Non-polar liquids will be miscible with another nonpolar liquid – Salad oil and motor oil • A non-polar liquid will not be miscible with a polar liquid – Oil and water • LIKE DISSOLVES LIKE!

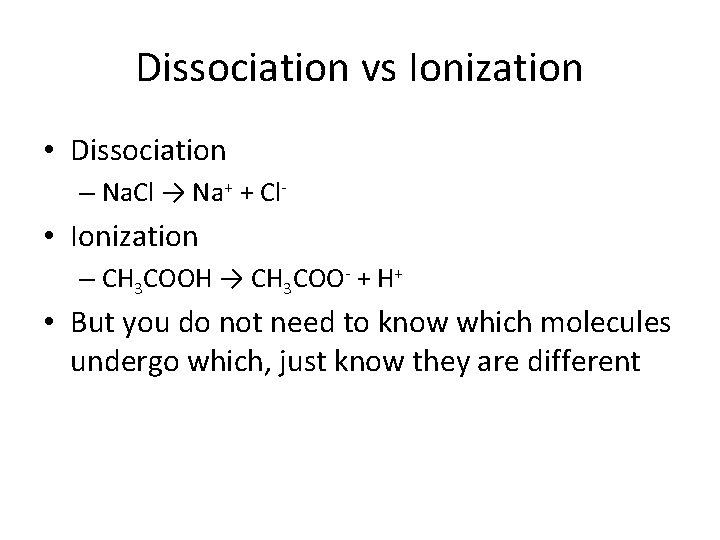
Some terms to know • Ionic solid – are crystals made up of ions and are charged • Molecular solid – are crystals made up of neutral molecules and are not charged • Dissociation – Reaction that separates existing ions in an ionic solid • Ionization – Reaction that breaks up a neutral molecule into ions

Dissociation vs Ionization • Dissociation – Na. Cl → Na+ + Cl- • Ionization – CH 3 COOH → CH 3 COO- + H+ • But you do not need to know which molecules undergo which, just know they are different

Practice - 1 • Page 210 - #28 -29

Ion Concentration • So just like in stoichiometry when reactants change into products. Solubility is like a reaction. • Except of it changing from reactants to products, we are changing it from solid to ions • When we put Na. Cl into water, it dissociates into Na+ and Cl– To write it in an equation, it becomes – Na. Cl → Na+ + Cl- • If we do it with Ca. Cl 2 and put it into water – Ca. Cl 2 → Ca 2+ and 2 Cl-

Ion Concentration • Remember, the coefficients just means moles. • Since the ions are in the same volume as the ionic salt, their concentration should be related • They are related by the coefficients • If the ionic salt has a certain concentration, you can do mole bridge conversion to find the ion concentrations

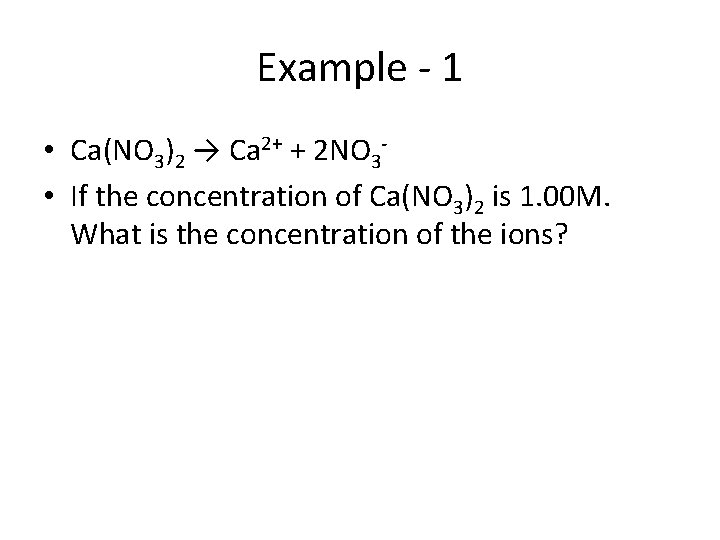
Example - 1 • Ca(NO 3)2 → Ca 2+ + 2 NO 3 • If the concentration of Ca(NO 3)2 is 1. 00 M. What is the concentration of the ions?

Example - 2 • 300. 0 m. L of 2. 00 M Na. OH is mixed with 600. 0 m. L of 5. 00 M Mg. I 2 • What is the concentration of each substance in the resulting solution? • What is the concentration of each ion in the resulting solution?

Example - 3 • 50. 0 m. L of 0. 250 M Al. Br 3 and 25. 0 m. L of 0. 300 M Ca. Br 2 are mixed together. • What is the resulting concentration of each solution? • What is the concentration of each ion?

Practice - 2 • Page 212 - #30 -38