SOLUTIONS If youre not part of the solution





























- Slides: 54

SOLUTIONS “If you’re not part of the solution, you’re part of the precipitate”

Properties of Solutions Definition of a Solution: a homogeneous mixture of substances in the same physical state v. What does Homogeneous mean? atoms, ions, or molecules of one substance spread uniformly throughout a second substance

Types of Solutions exist in all three states (solid, liquid and gas). u What is it called when metals are mixed to form a solid solution? alloy uex. brass = Cu/Zn; steel = iron + carbon u u What is an example of gases forming a solution? Air (is a homogeneous mixture) u The majority of this topic will be limited to only liquid solutions, in which a solid is dissolved in a liquid. • ex. salt water, antifreeze

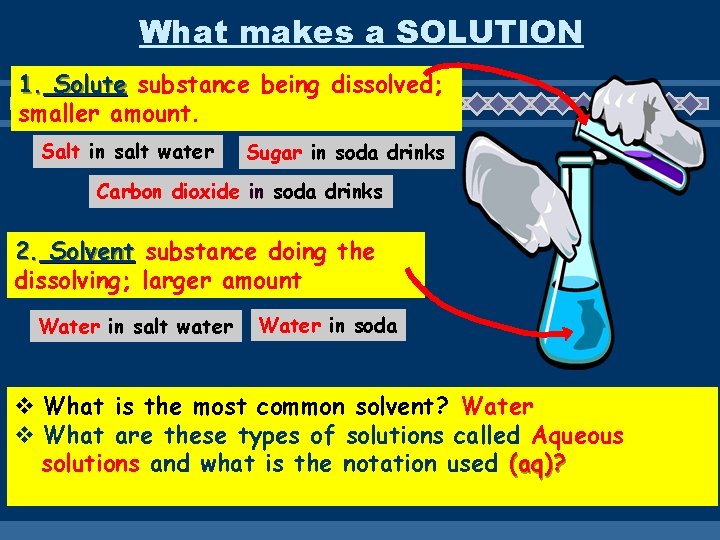
What makes a SOLUTION 1. Solute substance being dissolved; smaller amount. Salt in salt water Sugar in soda drinks Carbon dioxide in soda drinks 2. Solvent substance doing the dissolving; larger amount Water in salt water Water in soda v What is the most common solvent? Water v What are these types of solutions called Aqueous solutions and what is the notation used (aq)?

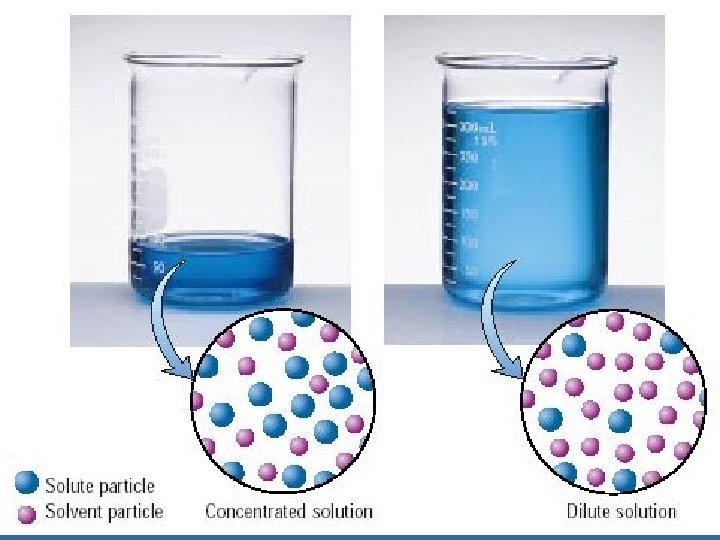
Concentrated vs. Dilute

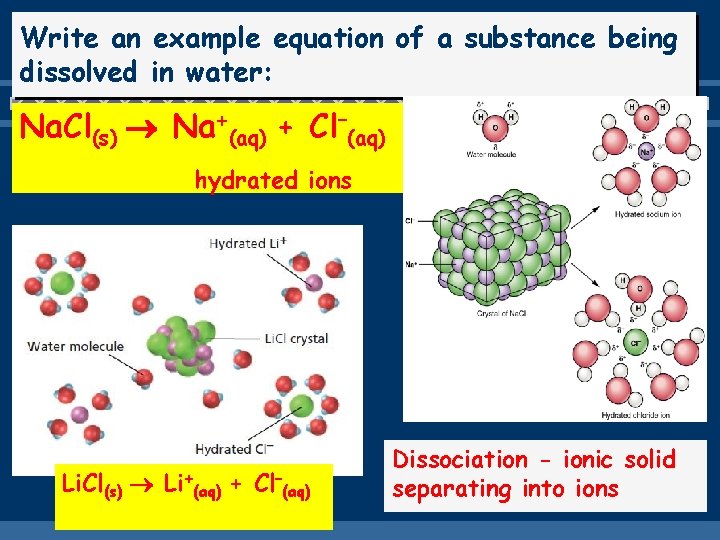
Write an example equation of a substance being dissolved in water: Na. Cl(s) Na+(aq) + Cl–(aq) hydrated ions Li. Cl(s) Li+(aq) + Cl–(aq) Dissociation - ionic solid separating into ions

5 Main points about all liquid solutions…. . 1. Solutions are homogeneous mixtures (aq) –known as true solutions 2. Solutions are clear & do not disperse light 3. Solutions can have color 4. Solutions will not settle upon standing 5. Solutions will pass through a filter

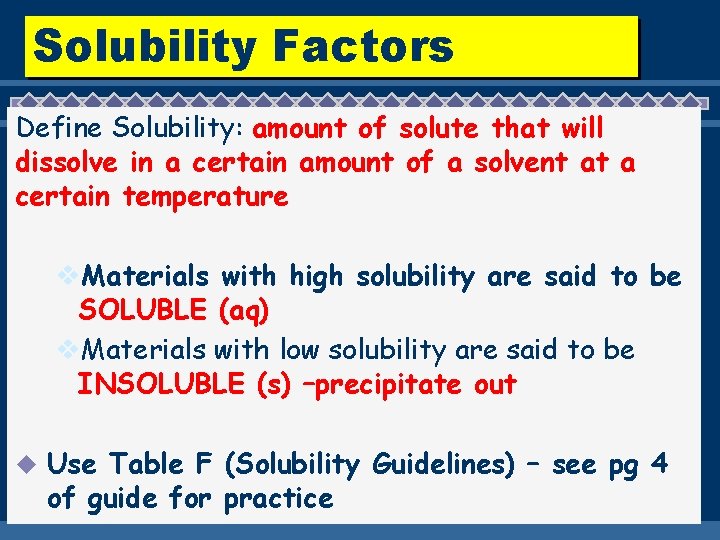
Solubility Factors Define Solubility: amount of solute that will dissolve in a certain amount of a solvent at a certain temperature v. Materials with high solubility are said to be SOLUBLE (aq) v. Materials with low solubility are said to be INSOLUBLE (s) –precipitate out u Use Table F (Solubility Guidelines) – see pg 4 of guide for practice

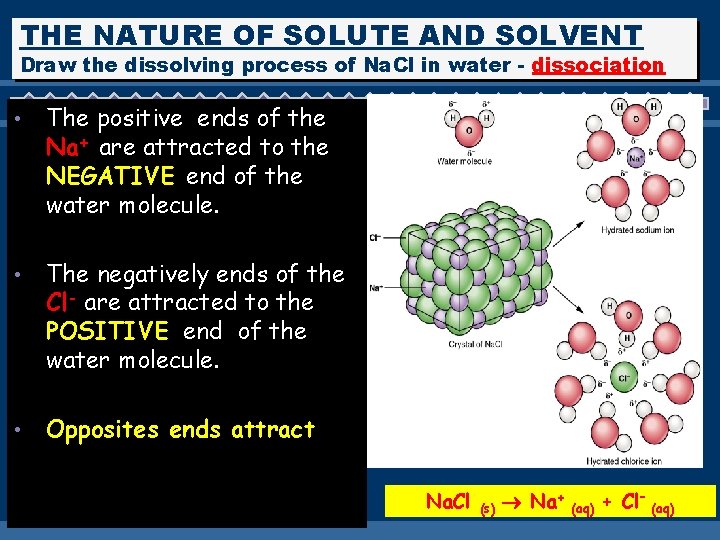
THE NATURE OF SOLUTE AND SOLVENT Draw the dissolving process of Na. Cl in water - dissociation • The positive ends of the Na+ are attracted to the NEGATIVE end of the water molecule. • The negatively ends of the Cl- are attracted to the POSITIVE end of the water molecule. • Opposites ends attract Na. Cl (s) Na+ (aq) + Cl– (aq)

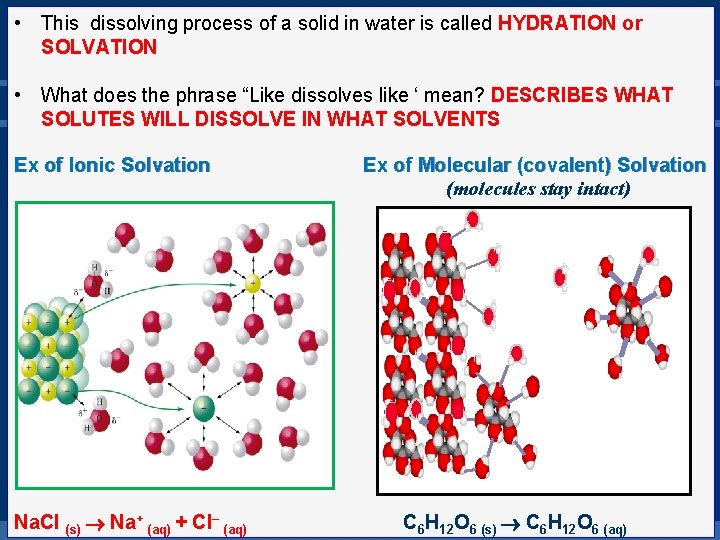
• This dissolving process of a solid in water is called HYDRATION or SOLVATION • What does the phrase “Like dissolves like ‘ mean? DESCRIBES WHAT SOLUTES WILL DISSOLVE IN WHAT SOLVENTS Ex of Ionic Solvation Na. Cl (s) Na+ (aq) + Cl– (aq) Ex of Molecular (covalent) Solvation (molecules stay intact) C 6 H 12 O 6 (s) C 6 H 12 O 6 (aq)

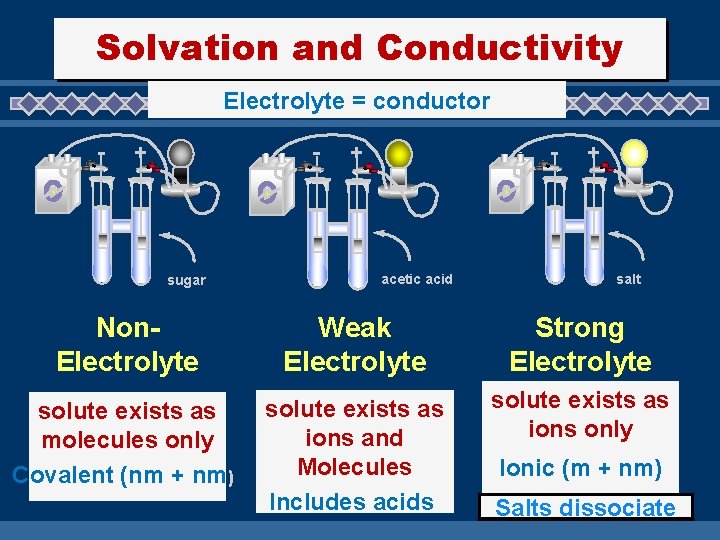
Solvation and Conductivity Electrolyte = conductor - - + sugar - + acetic acid + salt Non. Electrolyte Weak Electrolyte Strong Electrolyte solute exists as molecules only Covalent (nm + nm) solute exists as ions and Molecules Includes acids solute exists as ions only Ionic (m + nm) Salts dissociate

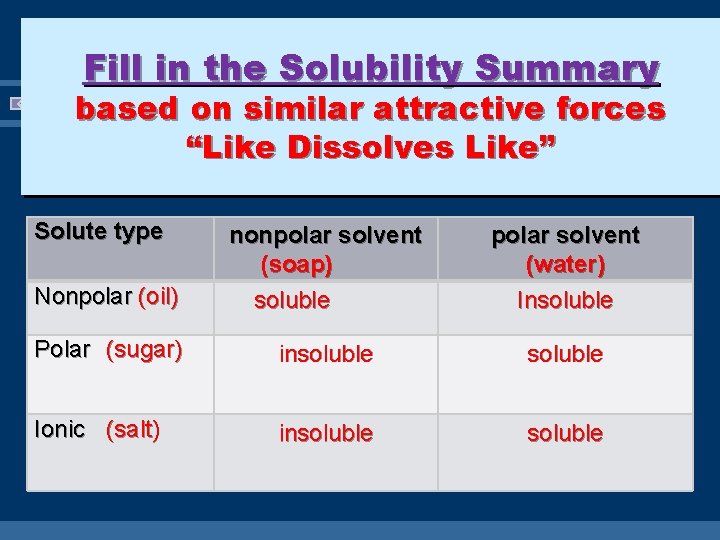
Fill in the Solubility Summary based on similar attractive forces “Like Dissolves Like” Solute type Nonpolar (oil) nonpolar solvent (soap) soluble polar solvent (water) Insoluble Polar (sugar) insoluble Ionic (salt) insoluble

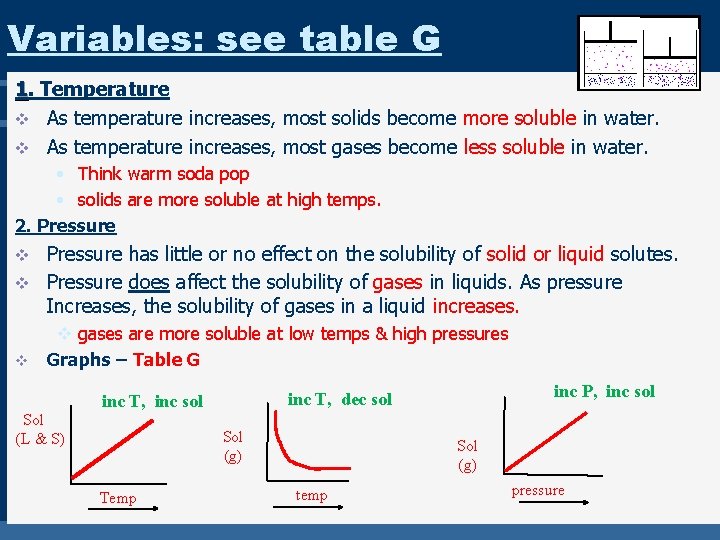
Variables: see table G 1. Temperature v As temperature increases, most solids become more soluble in water. v As temperature increases, most gases become less soluble in water. • Think warm soda pop • solids are more soluble at high temps. 2. Pressure has little or no effect on the solubility of solid or liquid solutes. v Pressure does affect the solubility of gases in liquids. As pressure Increases, the solubility of gases in a liquid increases. v v v gases are more soluble at low temps & high pressures Graphs – Table G Sol (L & S) Sol (g) Temp inc P, inc sol inc T, dec sol inc T, inc sol Sol (g) temp pressure

Solution Formation u A cube of sugar in cold tea dissolves slowly. . u Granulated sugar u dissolves in cold water more quickly than a sugar cube, especially with stirring Granulated sugar dissolves very quickly in hot tea

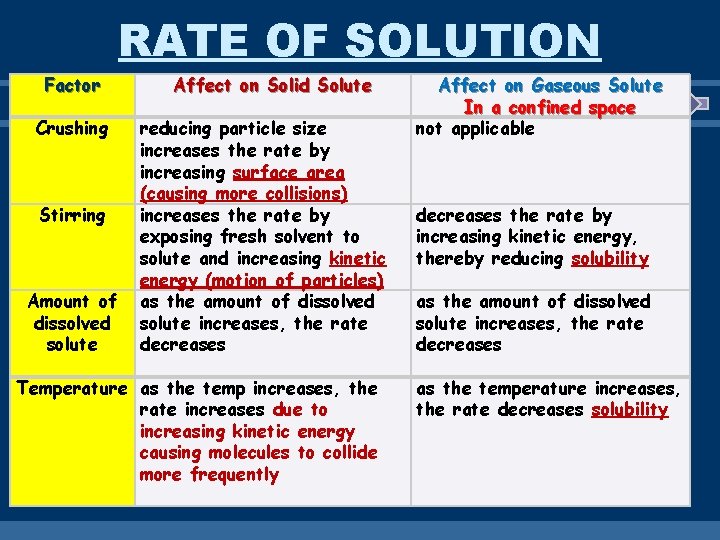
RATE OF SOLUTION Factor Crushing Stirring Amount of dissolved solute Affect on Solid Solute reducing particle size increases the rate by increasing surface area (causing more collisions) increases the rate by exposing fresh solvent to solute and increasing kinetic energy (motion of particles) as the amount of dissolved solute increases, the rate decreases Temperature as the temp increases, the rate increases due to increasing kinetic energy causing molecules to collide more frequently Affect on Gaseous Solute In a confined space not applicable decreases the rate by increasing kinetic energy, thereby reducing solubility as the amount of dissolved solute increases, the rate decreases as the temperature increases, the rate decreases solubility

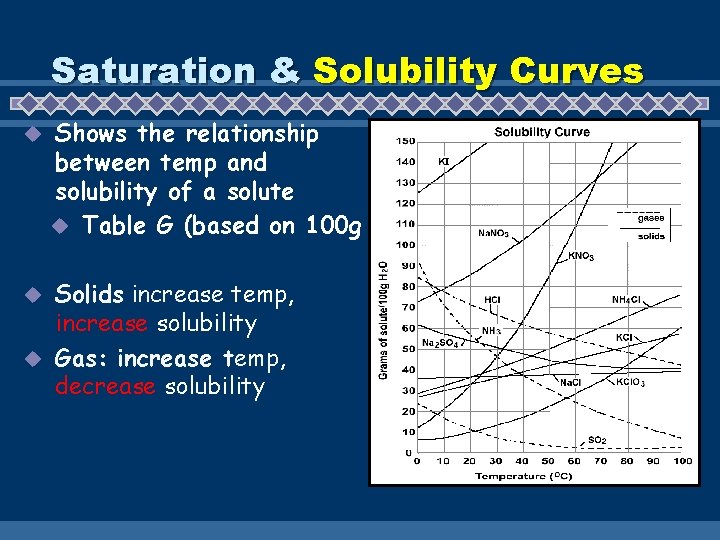
Saturation & Solubility Curves u Shows the relationship between temp and solubility of a solute u Table G (based on 100 g Solids increase temp, increase solubility u Gas: increase temp, decrease solubility u

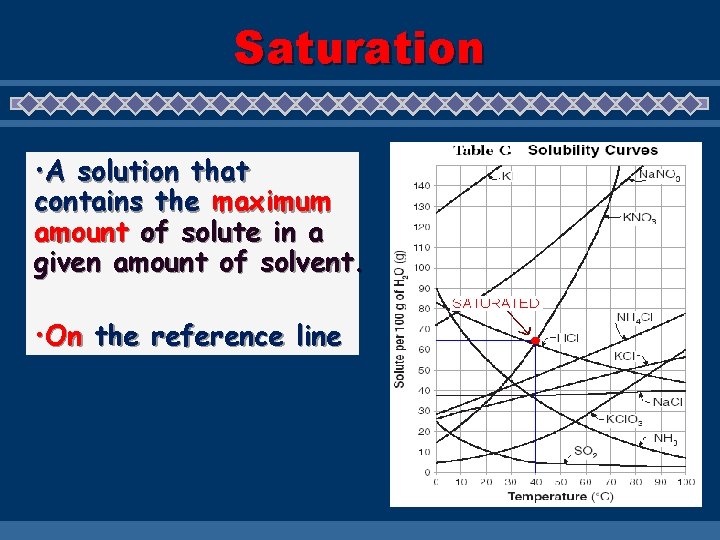
Saturation • A solution that contains the maximum amount of solute in a given amount of solvent. • On the reference line

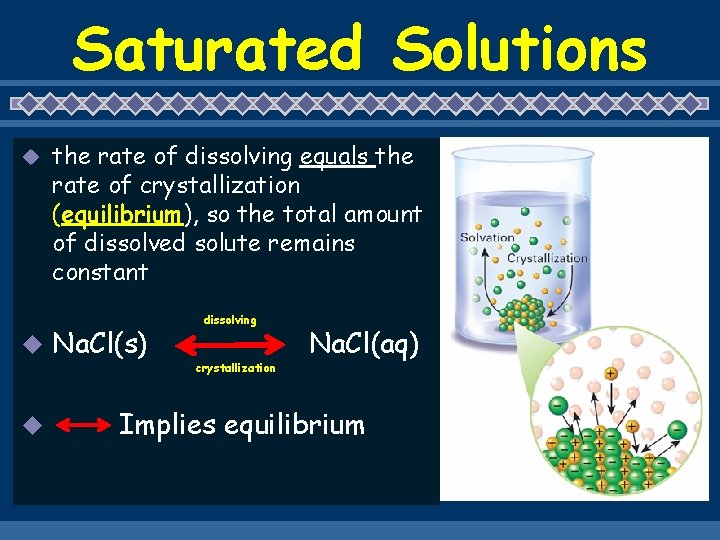
Saturated Solutions u u u the rate of dissolving equals the rate of crystallization (equilibrium), so the total amount of dissolved solute remains constant Na. Cl(s) dissolving crystallization Na. Cl(aq) Implies equilibrium

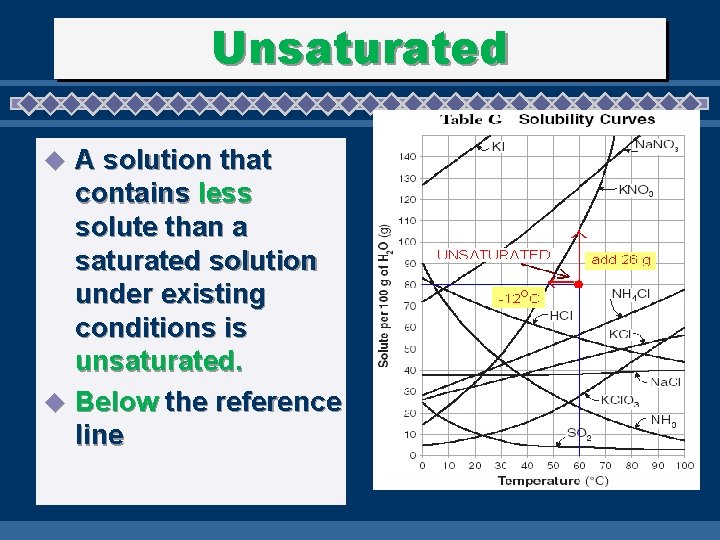
Unsaturated A solution that contains less solute than a saturated solution under existing conditions is unsaturated. u Below the reference line u

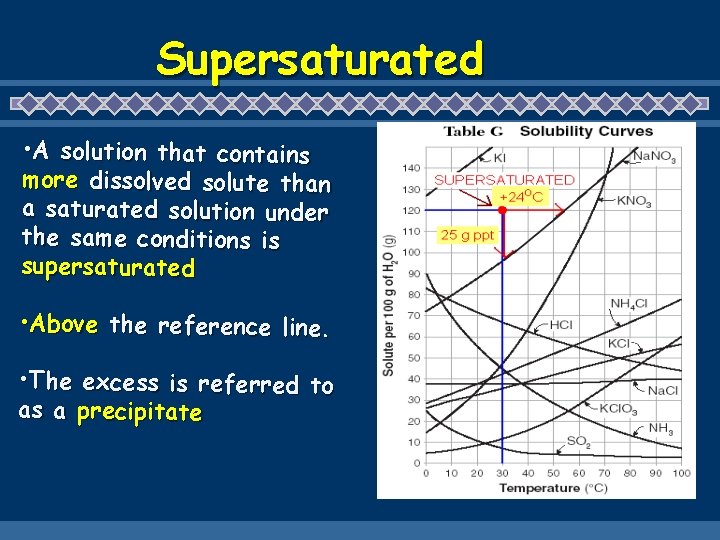
Supersaturated • A solution that contains more dissolved solute than a saturated solution under the same conditions is supersaturated • Above the reference line. • The excess is referred to as a precipitate

Supersaturated u A supersaturated solution is clear before a seed crystal is added. u Crystals begin to form in the solution immediately after the addition of a seed crystal. u Excess solute crystallizes rapidly

Practice Question #1 1) According to Reference Table G, which compound solubility decreases most rapidly as the temperature changes from 10°C to 70°C? a) NH 4 Cl b) NH 3 c) HCl d) KCl

Answer to problem #1 u Correct Answer Number: 2 u Explanation: See Ref. Table G. Notice the curves for choices 1 and 4 increase. Choice 2 and 3 both decrease but choice 2 (NH 3) decreases more than 50 degrees, while HCl decreases only about 16 degrees.

Molarity • The concentration of a solution is a measure of the amount of solute that is dissolved in a given quantity of solvent. – A dilute solution is one that contains a small amount of solute. – A concentrated solution contains a large amount of solute.

Concentrations of Solutions • Water must be tested continually to ensure that the concentrations of contaminants do not exceed established limits. These contaminants include metals, pesticides, bacteria, and even the by-products of water treatment. You will learn how solution concentrations are calculated.

Quantities in Solutions u The amount of solute in a solution. u Describing Concentration • % by mass - medicated creams • % by volume - rubbing alcohol • ppm, ppb - water contaminants • molarity - used by chemists • molality - used by chemists

Molarity • Molarity (M) is the number of moles of solute dissolved in one liter of solution. • To calculate the molarity of a solution, divide the moles of solute by the volume of the solution.

Molarity • To make a 0. 25 molar (0. 25 M) solution, first add ? mol of solute to a 0. 50 -L (500 m. L) volumetric flask half filled with distilled water.

Solve for Moles u Solute used is Na. Cl u We want to prepare 500 m. L of a 0. 25 Molar solution u Using the Molarity formula solve for moles of solute M = Moles of Solute Liters of Soln

Plug in Numbers u 0. 250 mol/Liter = x moles 0. 500 L # of Moles = 0. 250 mol/liter x 0. 500 L x = 0. 125 moles

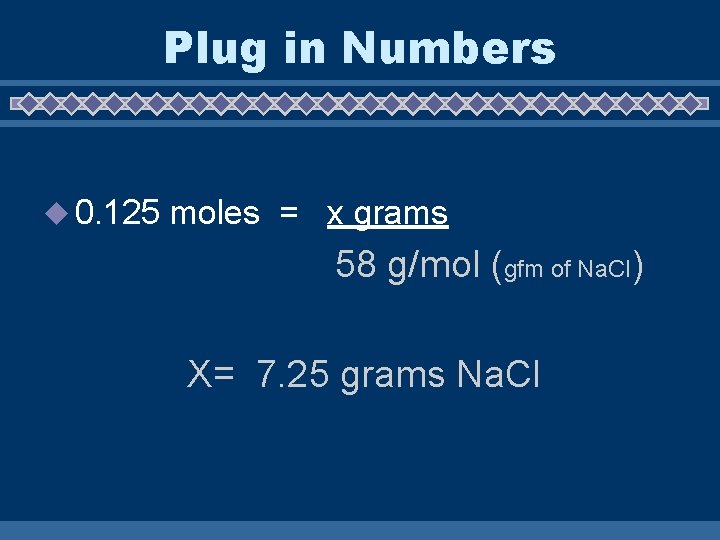
Convert Moles to Grams u 0. 125 moles Na. Cl ? Grams u Use Mole Conversion Formula u Moles = given mass gfm

Plug in Numbers u 0. 125 moles = x grams 58 g/mol (gfm of Na. Cl) X= 7. 25 grams Na. Cl

Molarity • Add 7. 25 grams of Na. Cl to water in flask • Swirl the flask carefully to dissolve the solute.

16. 2 Molarity • Fill the flask with water exactly to the 500 -m. L mark.

#1: How many moles of Na. OH are contained in 200 ml of 0. 1 M solution of Na. OH? 2) Which solution is the most concentrated? a) 1 mole of solute dissolved in 1 liter of solution? b) 2 moles of solute dissolved in 3 liters of solution? c) 6 moles of solute dissolved in 4 liters of solution? d) 4 moles of solute dissolved in 8 liters of solution?

Answer: u moles = (M)(L) u = (0. 1 M)(. 2 L) u =. 02 mol u u u Correct Answer Number: 3 Explanation: #3 (1. 5 M. ) is the most concentrated. Find the molarity ( moles of solute/liter of solution) for each answer. #1) 1 mole / 1 liter or 1 M. #2) 2 moles / 3 liters or 0. 67 M. #3) 6 moles / 4 liters or 1. 5 M. #4) 4 moles / 8 liters or 0. 5 M.

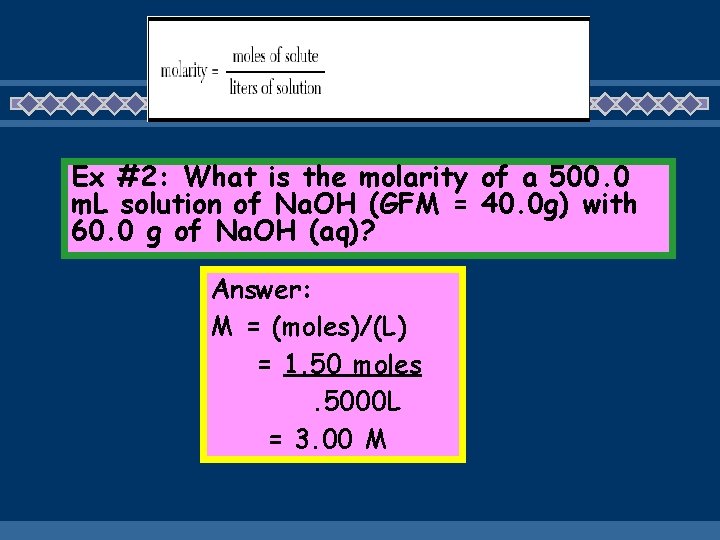
Ex #2: What is the molarity of a 500. 0 m. L solution of Na. OH (GFM = 40. 0 g) with 60. 0 g of Na. OH (aq)? Answer: M = (moles)/(L) = 1. 50 moles. 5000 L = 3. 00 M

Different style problem 1) Which solution is the most concentrated? a) b) c) d) 1 mole of solute dissolved in 1 liter of solution? 2 moles of solute dissolved in 3 liters of solution? 6 moles of solute dissolved in 4 liters of solution? 4 moles of solute dissolved in 8 liters of solution?

Answer to different style problem u u Correct Answer Number: 3 Explanation: #3 (1. 5 M. ) is the most concentrated. Find the molarity ( moles of solute/liter of solution) for each answer. #1) 1 mole / 1 liter or 1 M. #2) 2 moles / 3 liters or 0. 67 M. #3) 6 moles / 4 liters or 1. 5 M. #4) 4 moles / 8 liters or 0. 5 M.

16. 2 Percent Solutions • Concentration in Percent (Volume/Volume)

16. 2 Percent Solutions • Isopropyl alcohol (2 -propanol) is sold as a 91% solution. This solution consist of 91 m. L of isopropyl alcohol mixed with enough water to make 100 m. L of solution.

Percent Solutions

Percent Solutions

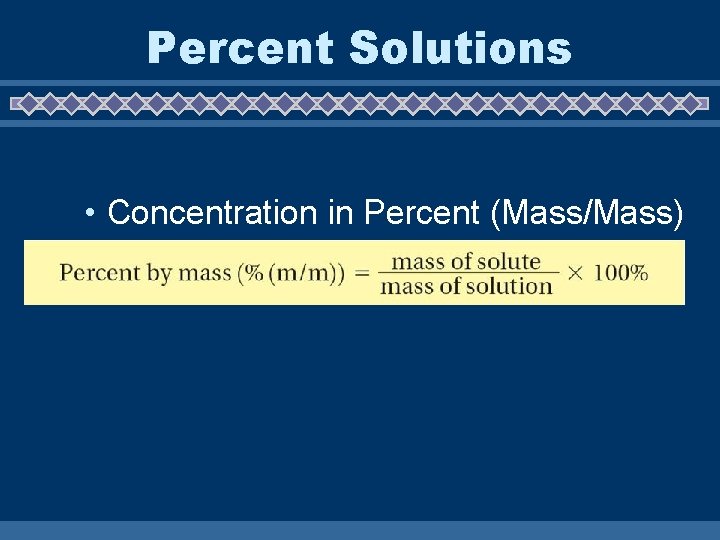
16. 2 Percent Solutions • Concentration in Percent (Mass/Mass)

u A 50. 0 gram sample of a solution is evaporated and found to contain 0. 100 grams of sodium chloride. What is the percent by mass of sodium chloride in the solution? % mass = 0. 100 g X 100 50. 0 g = 0. 200%

u 100. 0 grams of water is evaporated analyzed for lead. 0. 00010 grams of lead ions are found. What is the concentration of the lead, in parts per million? ppm = grams of solute x 1, 000 grams of solution = (0. 00010 g) x 1, 000 100. 00010 g =. 9 ppm • If the legal limit for lead in the water is 3. 0 ppm, then the water sample is within the legal limits (it’s safe and ok)

16. 3 u Colligative Properties of Solutions The wood frog is a remarkable creature because it can survive being frozen. Scientists believe that a substance in the cells of this frog acts as a natural antifreeze, which prevents the cells from freezing. You will discover how a solute can change the freezing point of a solution.

Colligative Properties u depend on the number of particles (molality) rather than the nature of the particles in the solution. u Boiling point, freezing point, vapor pressure and osmotic pressure are some of the properties affected.

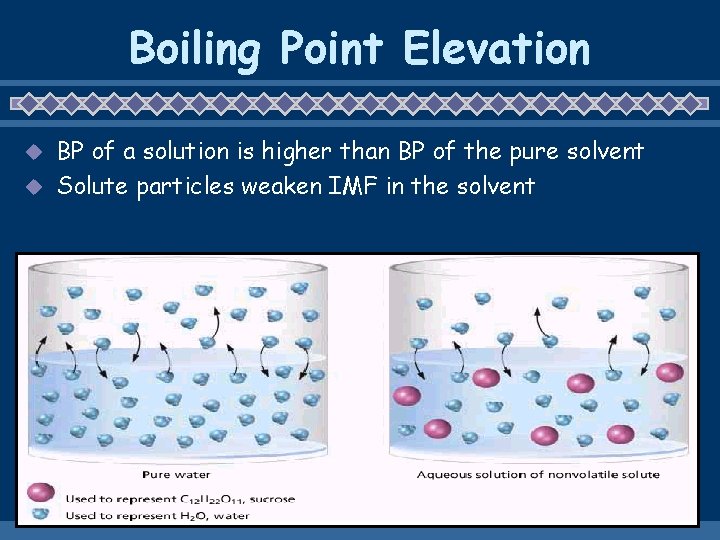
Boiling Point Elevation BP of a solution is higher than BP of the pure solvent u Solute particles weaken IMF in the solvent u

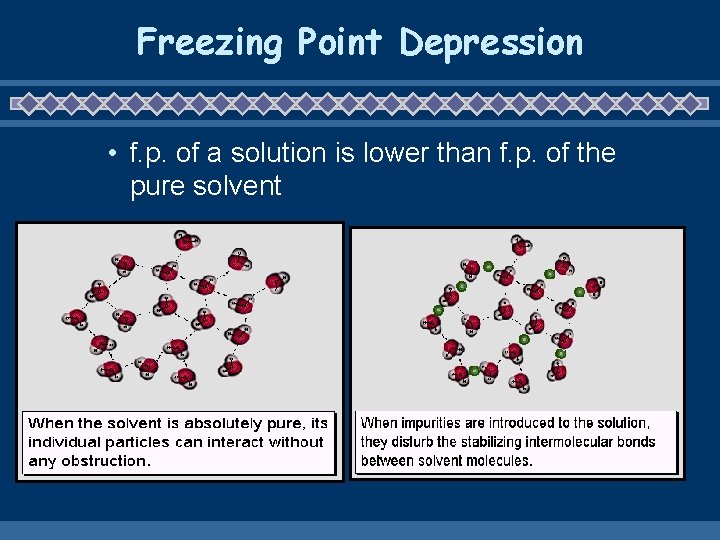
Freezing Point Depression • f. p. of a solution is lower than f. p. of the pure solvent

Applications • salting icy roads • making ice cream • antifreeze • cars (-64°C to 136°C) • fish & insects

Colligative Properties con’t. • # of Particles • Nonelectrolytes (covalent) – remain intact when dissolved – 1 particle • Electrolytes (ionic) – dissociate into ions when dissolved – 2 or more particles

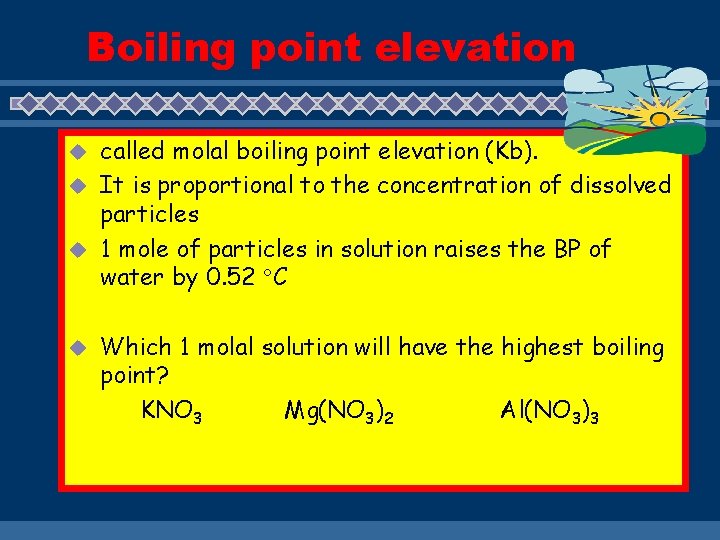
Boiling point elevation called molal boiling point elevation (Kb). u It is proportional to the concentration of dissolved particles u 1 mole of particles in solution raises the BP of water by 0. 52 C u u Which 1 molal solution will have the highest boiling point? KNO 3 Mg(NO 3)2 Al(NO 3)3

Freezing Point Depression called molal freezing point depression (Kf). u 1 mole of particles in solution depresses the FP of water by 1. 86 C u u What will depress the FP of water the most…. Al. Cl 3 or Mg. Cl 2? Why? What would the new FP be?