Solutions I III Colligative Properties II III C







- Slides: 10

Solutions I III. Colligative Properties II III C. Johannesson

A. Definition u Colligative Property • property that depends on the concentration (# of moles) of solute particles, not their identity (size, mass) C. Johannesson

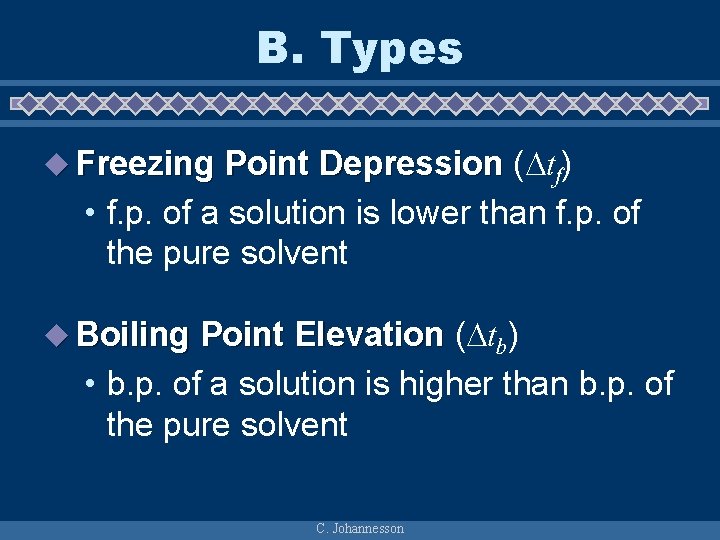
B. Types u Freezing Point Depression ( tf) • f. p. of a solution is lower than f. p. of the pure solvent u Boiling Point Elevation ( tb) • b. p. of a solution is higher than b. p. of the pure solvent C. Johannesson

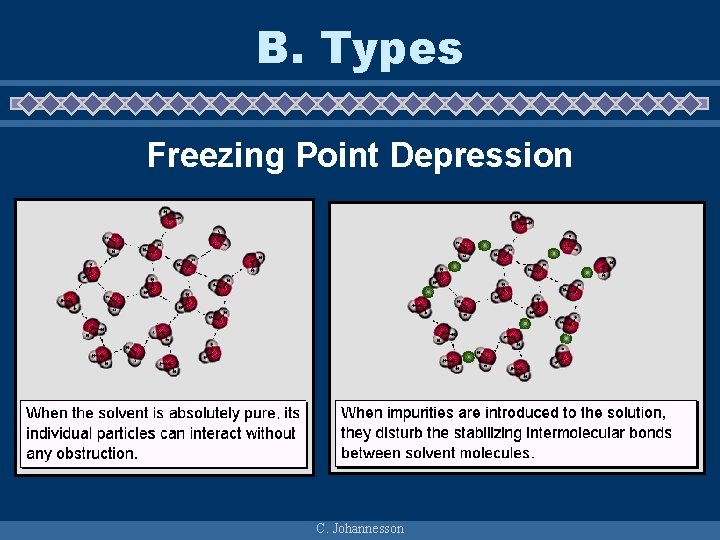
B. Types Freezing Point Depression C. Johannesson

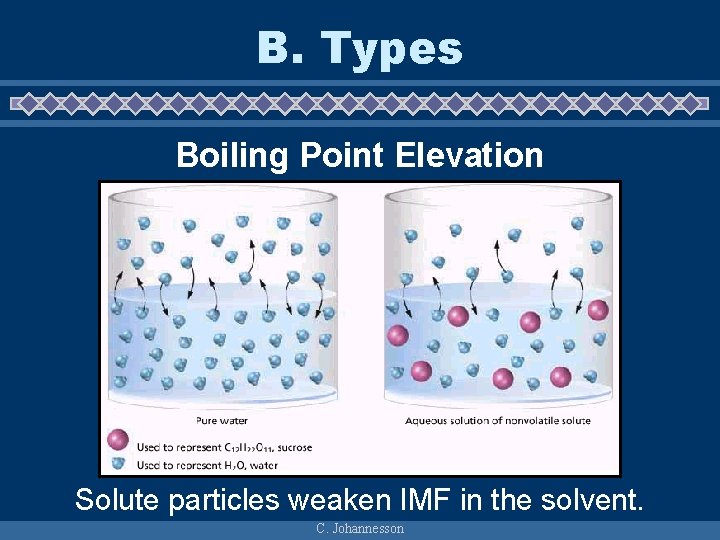
B. Types Boiling Point Elevation Solute particles weaken IMF in the solvent. C. Johannesson

B. Types u Applications • salting icy roads • making ice cream • antifreeze • cars (-64°C to 136°C) • fish & insects C. Johannesson

C. Calculations t = k · m · n t: change in temperature (°C) k: constant based on the solvent (°C·kg/mol) (pg 500, 502 or 975) m: molality (m) n: # of particles C. Johannesson

C. Calculations u # of Particles • Nonelectrolytes (covalent) • remain intact when dissolved • 1 particle • Electrolytes (ionic) • dissociate into ions when dissolved • 2 or more particles C. Johannesson

C. Calculations u Find the freezing point of a saturated solution of Na. Cl containing 28 g Na. Cl in 100. m. L water. GIVEN: f. p. = 0. 00°C tf = ? kf = 1. 86°C·kg/mol WORK: m = 0. 48 mol ÷ 0. 100 kg tf = (1. 86°C·kg/mol)(4. 8 m)(2) m = 4. 8 m n=2 tf = kf · m · n f. p. = 0. 00°C - 18°C tf = 18°C f. p. = -18°C C. Johannesson

C. Calculations u At what temperature will a solution that is composed of 0. 73 moles of glucose in 225 g of phenol boil? GIVEN: WORK: b. p. = 181. 8°C m = 0. 73 mol ÷ 0. 225 kg tb = ? tb = (3. 60°C·kg/mol)(3. 2 m)(1) kb = 3. 60°C·kg/mol tb = 11. 4°C m = 3. 2 m b. p. = 181. 8°C + 11. 4°C n=1 b. p. = 193°C tb = kb · m · n C. Johannesson