SOLUTIONS Homogeneous mixture of very small particles atoms


























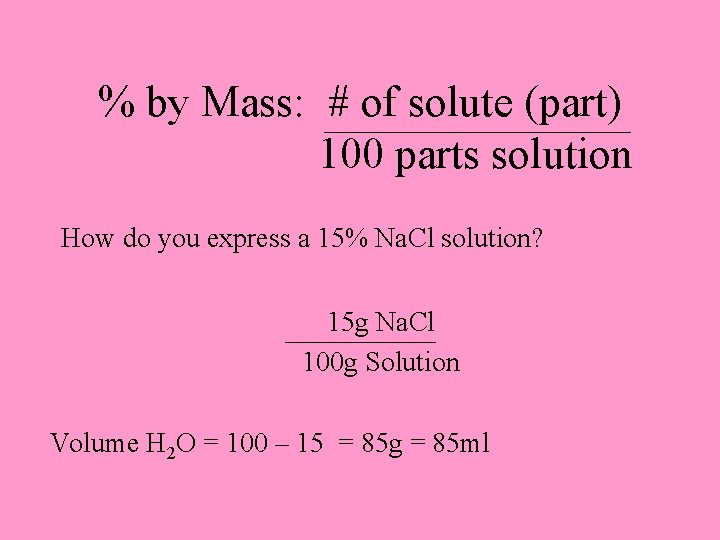
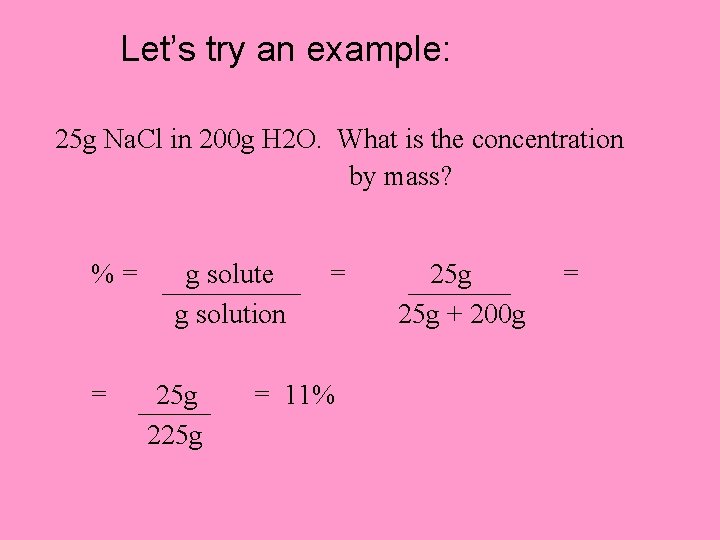



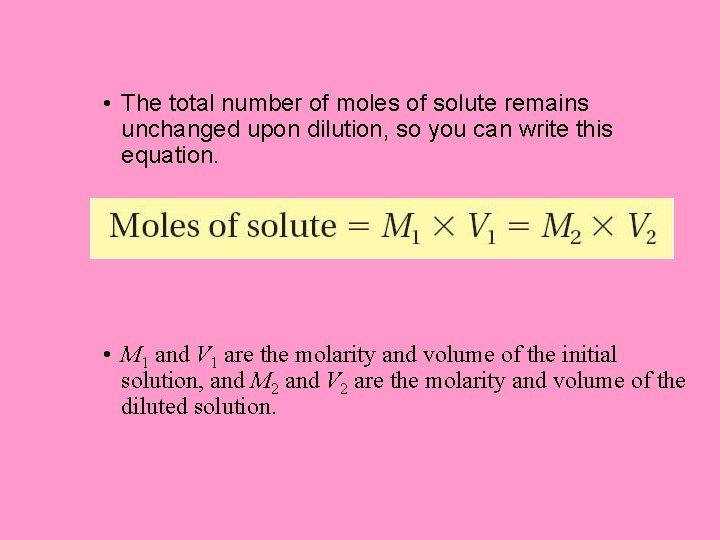










- Slides: 63

SOLUTIONS • Homogeneous mixture of very small particles (atoms, ions, molecules) • Sample from 1 area will have the same % solute as from another area • The dissolved particles will not come out of solution (assuming the solution is covered) • A solution is clear & transparent (particles are too small to see)

• A solution is considered a single phase (aqueous) Ex: Na. Cl + H 2 O Na. Cl (aq) CO 2 + H 2 O “Soda Water” • A solution will pass through a filter (filtration will NOT separate a solution)

Definitions: SOLUTE • What is dissolved • Smaller amount • Ex: salt SOLVENT • What is doing the dissolving • Larger amount • Ex: Water Solution = solute + solvent

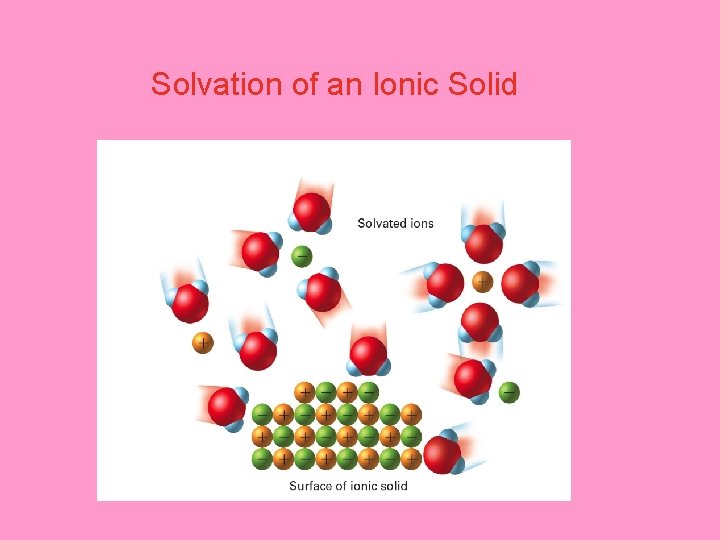
What happens in the solution process? – As individual solute ions break away from the crystal, the negatively and positively charged ions become surrounded by solvent molecules, and the ionic crystal dissolves. • The process is called solvation.

Solvation of an Ionic Solid

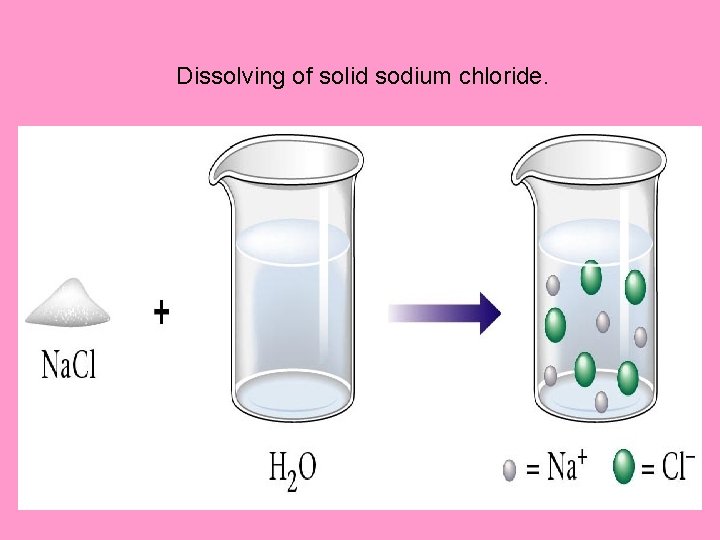
Dissolving of solid sodium chloride.

DEFINITIONS: MISCIBLE: when 2 liquids dissolve each other ex: Juice & water IMMISCIBLE: when 2 liquids CANNOT dissolve each other ex: OIL & water

Oil and water do not mix. Oil and water are immiscible.

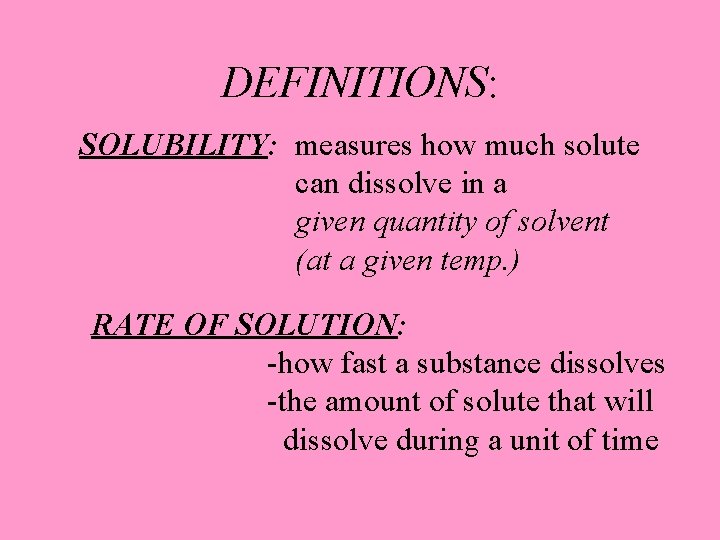
DEFINITIONS: SOLUBILITY: measures how much solute can dissolve in a given quantity of solvent (at a given temp. ) RATE OF SOLUTION: -how fast a substance dissolves -the amount of solute that will dissolve during a unit of time

Types of Solutions 1. Gaseous Solutions: -gases or vapors dissolved in one another EX: Air (O 2, N 2, H 2 O) 2. Liquid Solutions: - most common - liquid solvent in which a gas, liquid, or solid is dissolved Ex: sugar in water, soda, antifreeze

3. Solid Solutions: - mixtures of solids which are uniformly spread out ex: alloys: brass (Cu & Zn) Amalgam tooth filling (Hg)

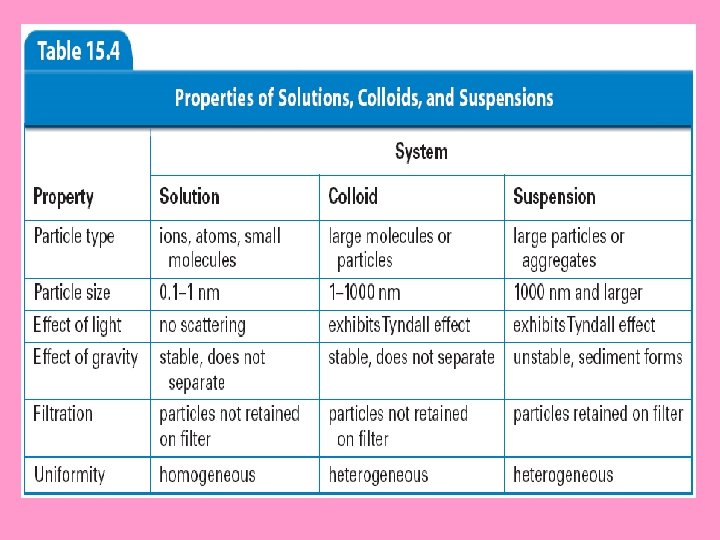
What is the difference between a suspension and a solution? • A suspension is a mixture from which particles settle out upon standing. • Suspensions are heterogenous because at least two substances can be clearly identified.

What distinguishes a colloid from a suspension and a solution? – Colloids have particles smaller than those in suspensions and larger than those in solutions.

• A colloid is a heterogeneous mixture containing particles that range in size from 1 nm to 1000 nm. • The particles in a colloid are spread throughout the dispersion medium.

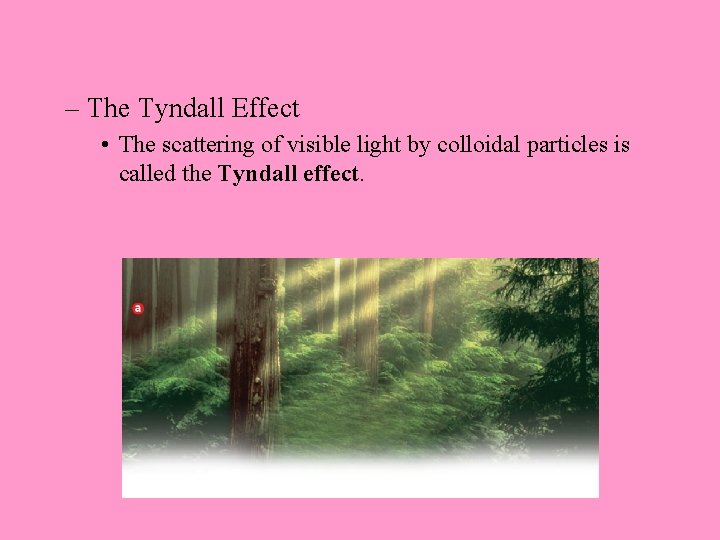
– The Tyndall Effect • The scattering of visible light by colloidal particles is called the Tyndall effect.

• Particles in colloids and suspensions reflect or scatter light in all directions. Solutions do not scatter light.

– Brownian Motion – The chaotic movement of colloidal particles, which was first observed by the Scottish botanist Robert Brown (1773– 1858), is called Brownian motion. – Brownian motion is caused by collisions of the molecules of the dispersion medium with the small, dispersed colloidal particles. – Coagulation – A colloidal system can be destroyed, or coagulated, by the addition of ions having a charge opposite to that of the colloidal particles. – The added ions neutralize the charged colloidal particles. The particles can clump together to form heavier aggregates and precipitate from the dispersion.

– Emulsions • An emulsion is a colloidal dispersion of a liquid in a liquid. An emulsifying agent is essential for the formation of an emulsion and for maintaining the emulsion’s stability. • Mayonnaise is heterogeneous mixture of oil and vinegar. Such a mixture would quickly separate without the presence of egg yolk, which is the emulsifying agent.


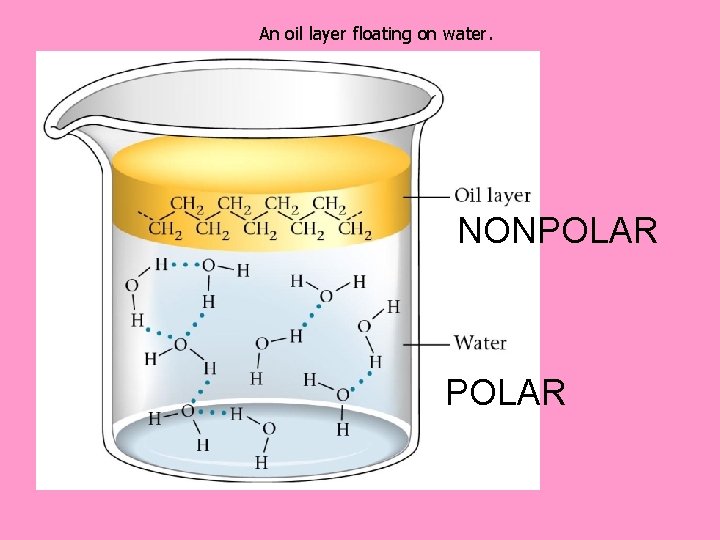
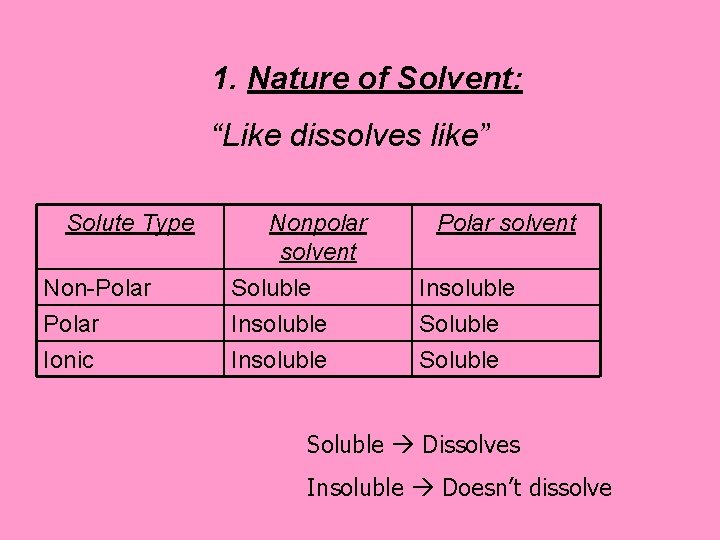
Factors Affecting Solubility: 1. Nature of Solvent: “Like dissolves like” Polar substances dissolve polar substances Nonpolar substances dissolve nonpolar substances This explains why water & oil do not mix

An oil layer floating on water. NONPOLAR

1. Nature of Solvent: “Like dissolves like” Solute Type Non-Polar Nonpolar solvent Soluble Insoluble Polar solvent Insoluble Soluble Ionic Insoluble Soluble Dissolves Insoluble Doesn’t dissolve

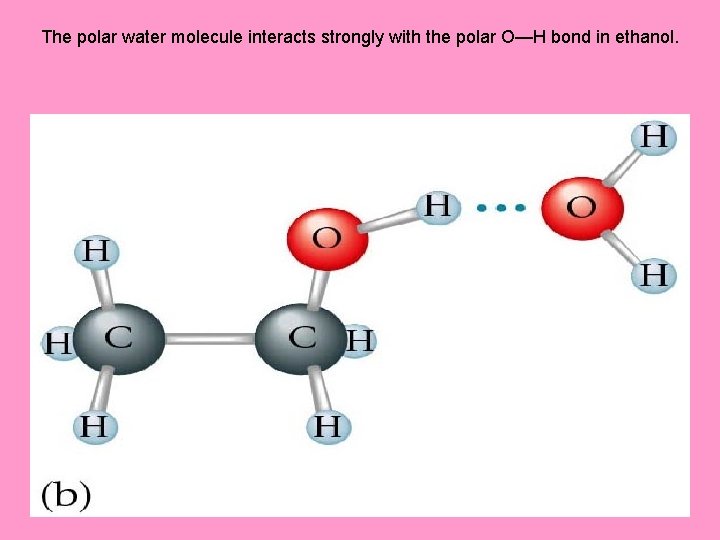
The polar water molecule interacts strongly with the polar O—H bond in ethanol.

2. Temperature Generally: increased temperature increases solubility (of a solid) -more solute can be dissolved at warmer temps Ex: cold tea vs. hot tea With a gas: solubility decreases with increased temps. Ex: cold vs. warm soda


– Temperature and Solution Formation • At higher temperatures, the kinetic energy of water molecules is greater than at lower temperatures, so they move faster. • As a result, the solvent molecules collide with the surface of the sugar crystals more frequently and with more force. • A cube of sugar in cold tea dissolves slowly.

3. Pressure • For solid & liquid solutes: pressure has NO effect on solubility • For Gaseous solutes: increased pressure increases solubility Henry’s Law: mass of dissolved gas is directly proportional to the pressure (in a given volume of liquid) * Not obeyed by gases that react with the solvent

Effervescence: The escape of gas from solution Ex: CO 2 from soda

4. Size of Particles: - for solid solutes increased surface area increases dissolving rate Ex: cubed sugar vs. Granulated sugar (smaller particle size has greater surface area) Ø if you increase the surface area the rate of dissolving increases (sugar cube versus granulated sugar), more collisions Granulated sugar dissolves more quickly than a sugar cube.

5. Stirring (Agitation): Stirring increases the rate of solution: There are more collisions between solute & solvent • Stirring speeds up the dissolving process because fresh solvent (the water in tea) is continually brought into contact with the surface of the solute (sugar).

6. Amount of Solute already dissolved -Little already dissolved: rapid dissolution -dissolving slows down when more solute is added

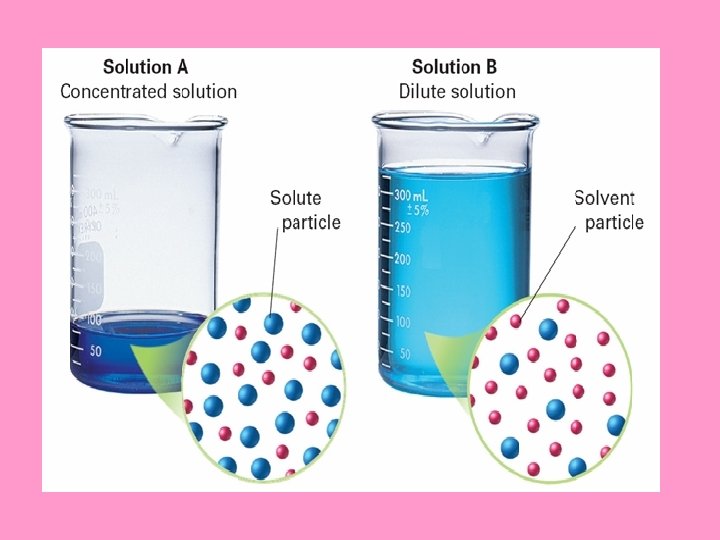
Expressing Solution Concentrations: • The concentration of a solution: measures the amount of solute that is dissolved in a given quantity of solvent Terms: Dilute vs. Concentrated dilute solution: contains a small amount of solute concentrate d solution: contains a large amount of solute


Concentrated vs. Dilute Let’s use tea as an example. If you like strong tea, then you like a concentrated solution; a relatively large amount of tea (solute) is dissolved in the water (solvent). If you like weak tea; this mixture is a dilute solution; relatively little tea is dissolved in the water, so the concentration is low (Dead Sea vs. Ocean)

Saturated/ Unsaturated/ Supersaturated (table G) • An unsaturated solution contains less solute than a saturated solution at a given temperature and pressure.

• A saturated solution contains the maximum amount of solute for a given quantity of solvent at a given temperature and pressure • In a saturated solution, the rate of dissolving equals the rate of crystallization, so the total amount of dissolved solute remains constant.

• A supersaturated solution contains more solute than it can theoretically hold at a given temperature. b. Crystals begin to form in the solution immediately after the addition of a seed crystal. A supersaturated solution is clear before a seed crystal is added. c. Excess solute crystallizes rapidly.

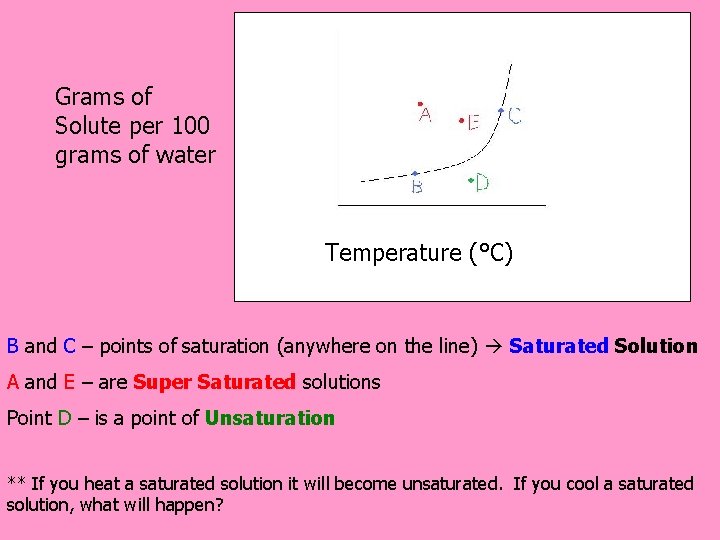
Grams of Solute per 100 grams of water Temperature (°C) B and C – points of saturation (anywhere on the line) Saturated Solution A and E – are Super Saturated solutions Point D – is a point of Unsaturation ** If you heat a saturated solution it will become unsaturated. If you cool a saturated solution, what will happen?

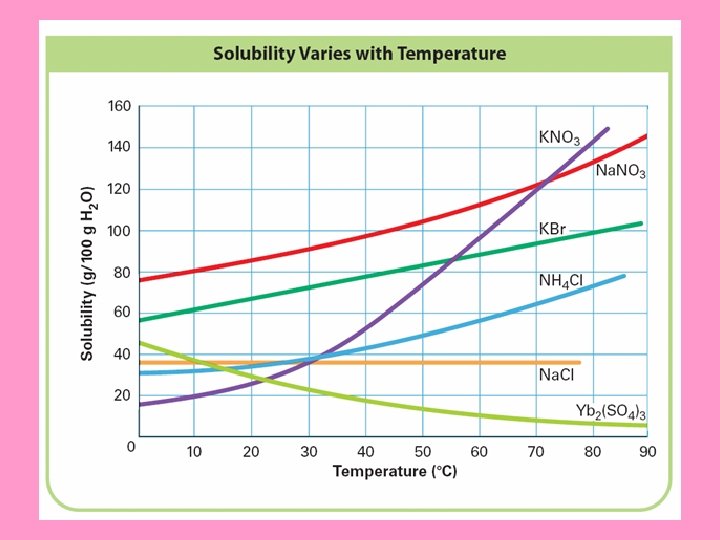
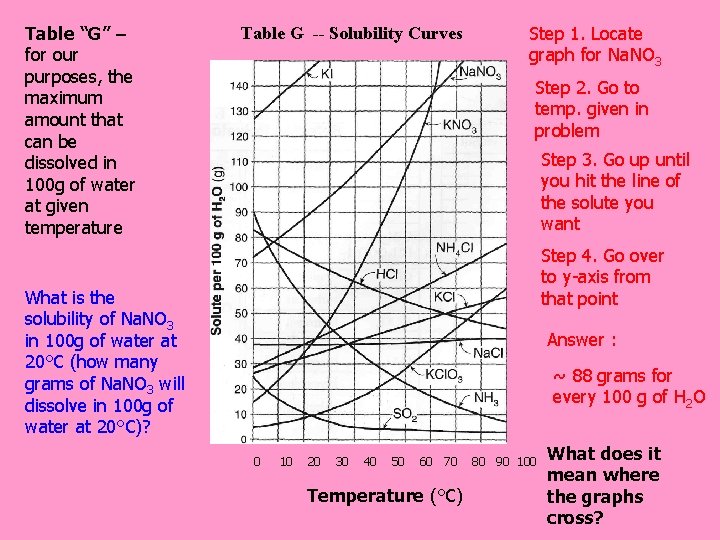
Table “G” – for our purposes, the maximum amount that can be dissolved in 100 g of water at given temperature Table G -- Solubility Curves Step 1. Locate graph for Na. NO 3 Step 2. Go to temp. given in problem Step 3. Go up until you hit the line of the solute you want Step 4. Go over to y-axis from that point What is the solubility of Na. NO 3 in 100 g of water at 20°C (how many grams of Na. NO 3 will dissolve in 100 g of water at 20°C)? Answer : ~ 88 grams for every 100 g of H 2 O 0 10 20 30 40 50 60 70 Temperature (°C) 80 90 100 What does it mean where the graphs cross?

Quantitative Methods: • % by mass • % by volume • Parts per million (ppm) • Molarity • Mass & volume relate through density • Formulas on table T

• Isopropyl alcohol (2 -propanol) is sold as a 91% solution. This solution consist of 91 m. L of isopropyl alcohol mixed with enough water to make 100 m. L of solution.
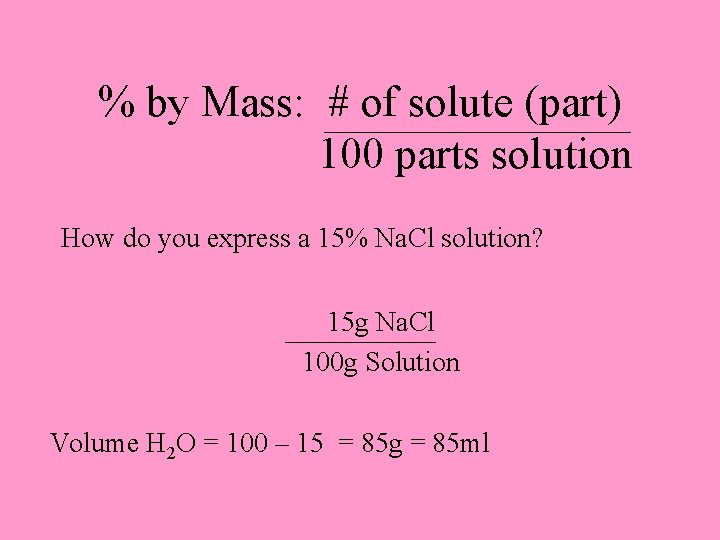
% by Mass: # of solute (part) 100 parts solution How do you express a 15% Na. Cl solution? 15 g Na. Cl 100 g Solution Volume H 2 O = 100 – 15 = 85 g = 85 ml
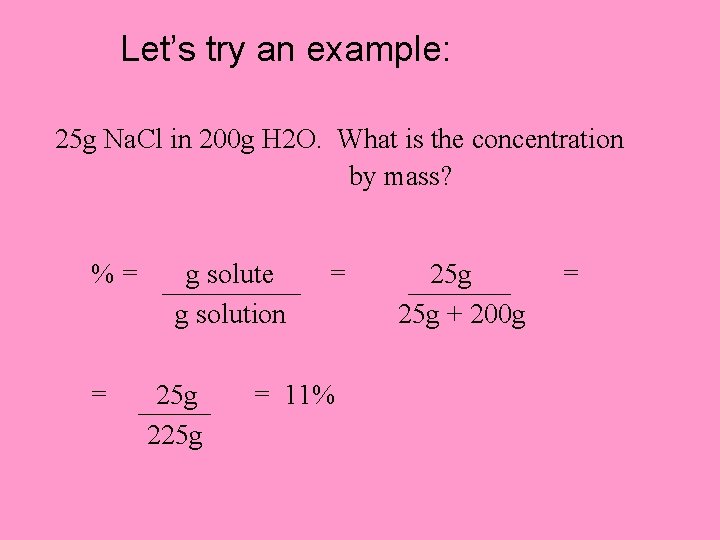
Let’s try an example: 25 g Na. Cl in 200 g H 2 O. What is the concentration by mass? %= = g solute g solution 25 g 225 g = = 11% 25 g + 200 g =

Let’s try some more: • What is the % by mass of 25 g Na. Cl in 90 ml of water? • A solution is 12% Na. Cl by mass. How many grams are there in 120 g of solution?

Parts Per Million (PPM) • Used for very dilute solutions Mass of solute in 1 million units of solution ppm = g solute X 1, 000 g solution Ex: An aqueous solution has 0. 004% Calcium by mass. How many ppm is this?

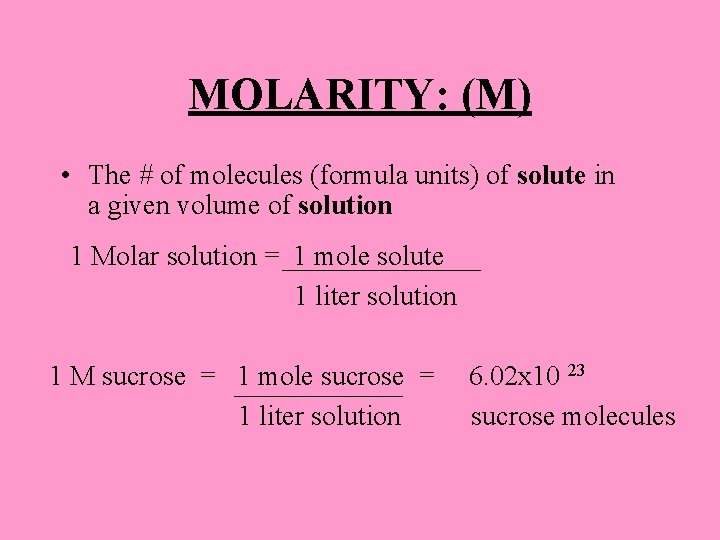
MOLARITY: (M) • The # of molecules (formula units) of solute in a given volume of solution 1 Molar solution = 1 mole solute 1 liter solution 1 M sucrose = 1 mole sucrose = 1 liter solution 6. 02 x 10 23 sucrose molecules

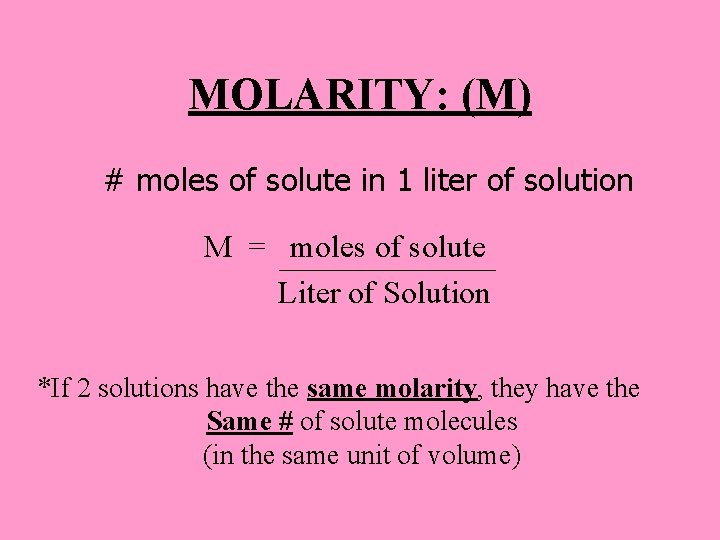
MOLARITY: (M) # moles of solute in 1 liter of solution M = moles of solute Liter of Solution *If 2 solutions have the same molarity, they have the Same # of solute molecules (in the same unit of volume)

• Making Dilutions – What effect does dilution have on the total moles of solute in a solution? – Diluting a solution reduces the number of moles of solute per unit volume, but the total number of moles of solute in solution does not change.
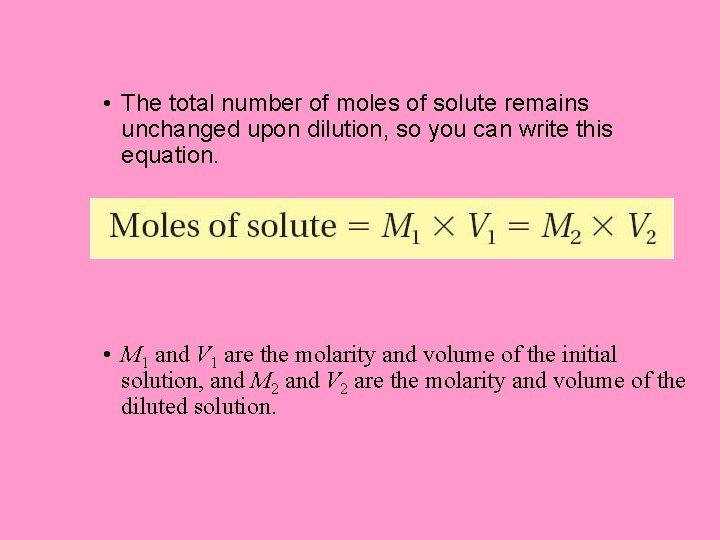
• The total number of moles of solute remains unchanged upon dilution, so you can write this equation. • M 1 and V 1 are the molarity and volume of the initial solution, and M 2 and V 2 are the molarity and volume of the diluted solution.

Colligative Properties of Solutions • A property that depends only upon the number of solute particles, and not upon their identity, is called a colligative property. – Three important colligative properties of solutions are • vapor-pressure lowering • boiling-point elevation • freezing-point depression

• The addition of a solute to water increases the boiling point of the water to more than 100°C and decreases the freezing point of the water to less than 0°C. v. The higher the concentration (more particles in solution), the greater the boiling point of water, and the lower the freezing point of water. v. This is the reason oceans do not freeze compared to lakes in the wintertime. This is why we put salt down to melt snow.

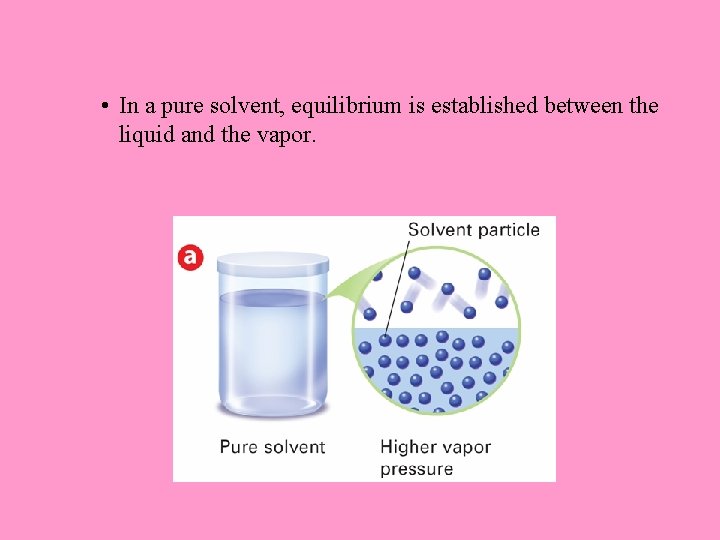
• In a pure solvent, equilibrium is established between the liquid and the vapor.

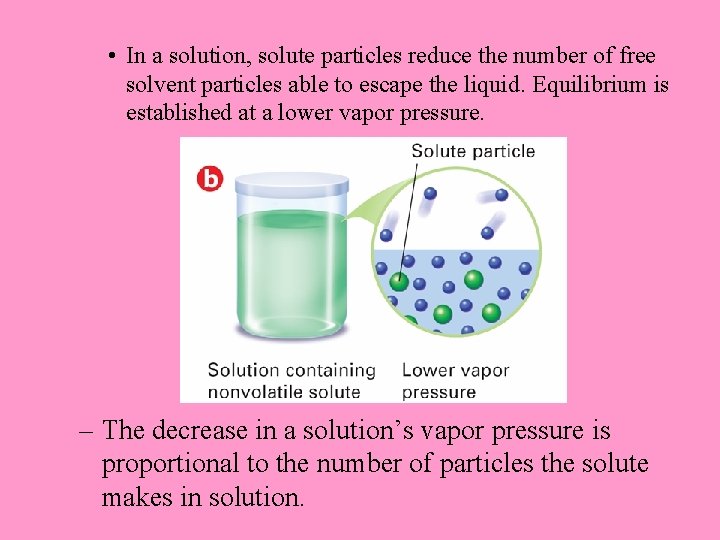
• In a solution, solute particles reduce the number of free solvent particles able to escape the liquid. Equilibrium is established at a lower vapor pressure. – The decrease in a solution’s vapor pressure is proportional to the number of particles the solute makes in solution.

• The difference in temperature between the freezing point of a solution and the freezing point of the pure solvent is the freezingpoint depression. – The magnitude of the freezing-point depression is proportional to the number of solute particles dissolved in the solvent and does not depend upon their identity.

• The freezing-point depression of aqueous solutions makes walks and driveways safer when people sprinkle salt on icy surfaces to make ice melt. • The melted ice forms a solution with a lower freezing point than that of pure water.

• Boiling-Point Elevation • The difference in temperature between the boiling point of a solution and the boiling point of the pure solvent is the boilingpoint elevation. • The same antifreeze added to automobile engines to prevent freeze-ups in winter, protects the engine from boiling over in summer.

• The magnitude of the boiling-point elevation is proportional to the number of solute particles dissolved in the solvent. • The boiling point of water increases by 0. 512°C for every mole of particles that the solute forms when dissolved in 1000 g of water.

• Three moles of glucose dissolved in water produces 3 mol of particles because glucose does not dissociate. Compared to organic compounds (carbon), inorganic compounds increase the boiling and decrease the freezing point more because inorganic compounds produce a greater number of particles in solution.

• Three moles of sodium chloride dissolved in water produce 6 mol of particles because each formula unit of Na. Cl dissociates into two ions.

• Three moles of calcium chloride dissolved in water produce 9 mol of particles because each formula unit of Ca. Cl 2 dissociates into three ions.

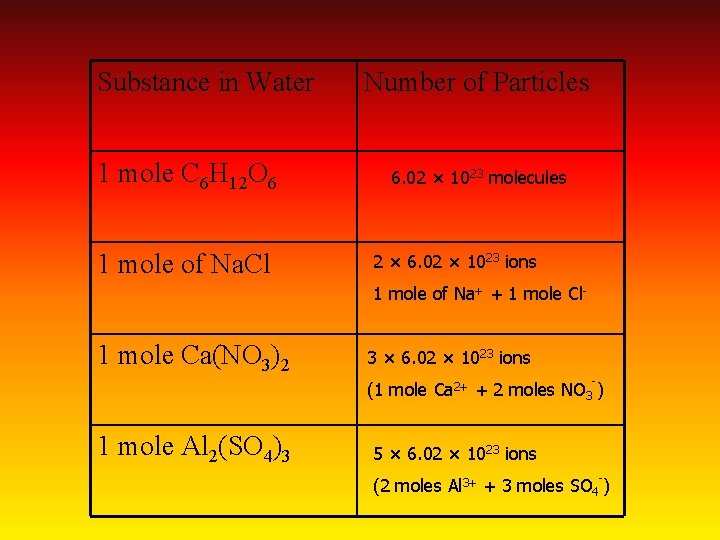
Substance in Water 1 mole C 6 H 12 O 6 1 mole of Na. Cl Number of Particles 6. 02 × 1023 molecules 2 × 6. 02 × 1023 ions 1 mole of Na+ + 1 mole Cl- 1 mole Ca(NO 3)2 3 × 6. 02 × 1023 ions - (1 mole Ca 2+ + 2 moles NO 3 ) 1 mole Al 2(SO 4)3 5 × 6. 02 × 1023 ions - (2 moles Al 3+ + 3 moles SO 4 )

One mole of particles lowers the freezing point of 1000 g of water by 1. 86°C. It also will elevate the boiling point of 1000 g H 2 O by. 52°C I have some ice on the steps of my house. I have 684 g of sugar C 12 H 22 O 11, in one bag, and 116 g of Na. Cl in another bag. Which will melt the ice best if I can empty the whole bag onto the steps?
