Solutions Formed when substances dissolve in other substances

Solutions • Formed when substances dissolve in other substances • Homogeneous mixture • Single phase • Remain mixed; particles do not settle out • Cannot be separated by filtration Episode 1001
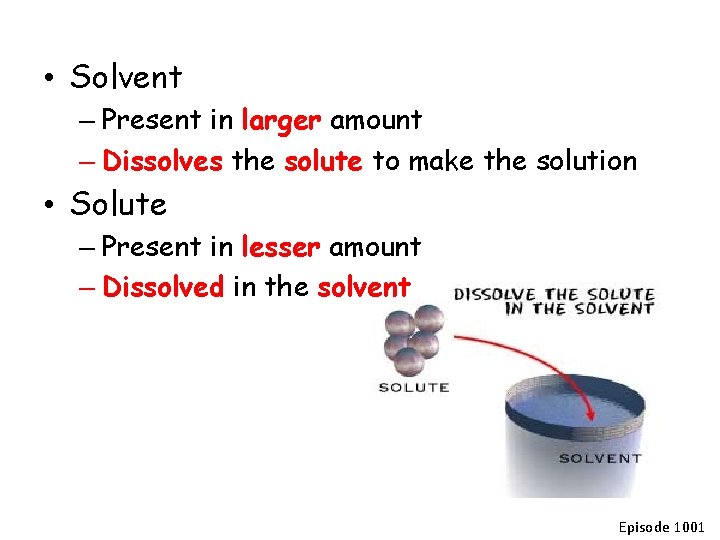
• Solvent – Present in larger amount – Dissolves the solute to make the solution • Solute – Present in lesser amount – Dissolved in the solvent Episode 1001
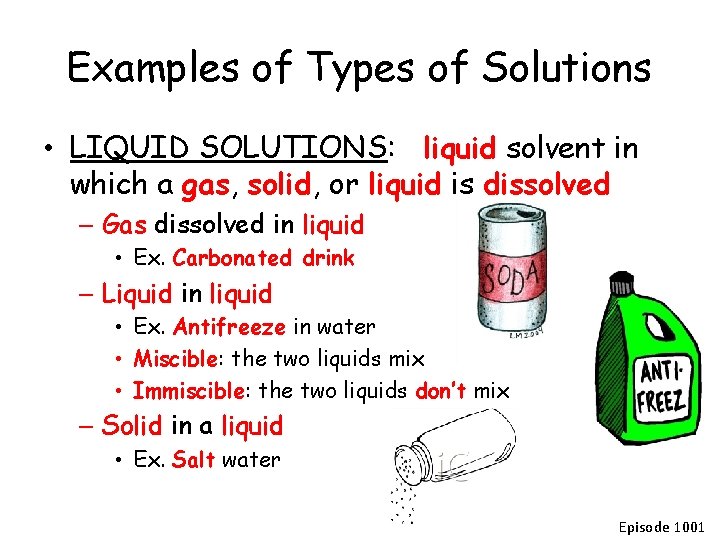
Examples of Types of Solutions • LIQUID SOLUTIONS: liquid solvent in which a gas, solid, or liquid is dissolved – Gas dissolved in liquid • Ex. Carbonated drink – Liquid in liquid • Ex. Antifreeze in water • Miscible: the two liquids mix • Immiscible: the two liquids don’t mix – Solid in a liquid • Ex. Salt water Episode 1001
• SOLID SOLUTIONS: • Alloys: solid mixtures of metals • (Brass is a mixture of copper and zinc) • GAS SOLUTIONS: • Gases dissolved in each other (air is most common example) • Aqueous: water is the solvent • Tincture: alcohol is the solvent Episode 1001

Suspensions • A heterogeneous mixture • Particles in the solvent are thousands of times larger than molecules and ions • Particles will settle out upon standing • Can be separated by filtration • Exhibit the Tyndall Effect – The scattering of light in all directions Episode 1001
Colloid • Particles are intermediate in size between those of suspensions and solutions • Particles do not settle out upon standing • Can not be separated by filtration • Exhibit the Tyndall Effect Episode 1001
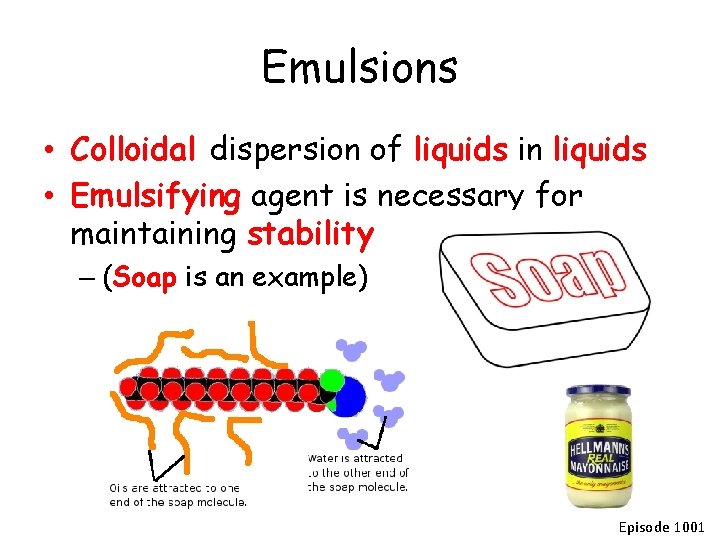
Emulsions • Colloidal dispersion of liquids in liquids • Emulsifying agent is necessary for maintaining stability – (Soap is an example) Episode 1001
• Electrolytes: • Dissolve in water to form a solution that conducts electric current • Nonelectrolytes: • Dissolves in water to form a solution that does not conduct electric current Episode 1001
Factors Affecting the Rate of Solution • Surface area: – Increasing the surface area of the solute by crushing speeds up dissolving by increasing the number of collisions between solvent and the solute surface • Agitation: – Stirring or shaking helps to disperse solute particles, increasing the number of collisions between solvent and the solute particles • Heating: – Increases the average kinetic energy of the solvent molecules so that the collisions between the solvent molecules and the solute are more frequent Episode 1001
- Slides: 9