Solutions Definitions Solution homogeneous mixture Solute substance that
Solutions

Definitions • Solution - homogeneous mixture Solute - substance that dissolves in a solvent and is said to be soluble Solvent - present in greater amount and dissolves solute

Universal Solvent is Water b/c almost everything dissolves in water Most common solvent among liquids is water Solute - KMn. O 4 Solvent - H 2 O
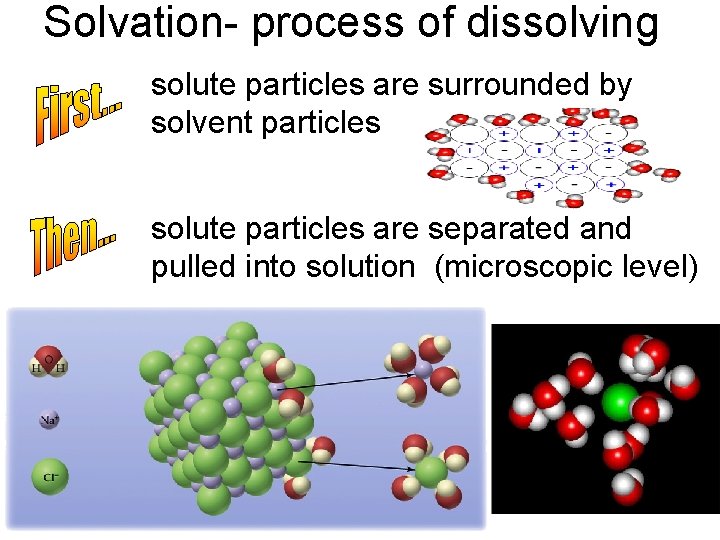
Solvation- process of dissolving solute particles are surrounded by solvent particles solute particles are separated and pulled into solution (microscopic level)
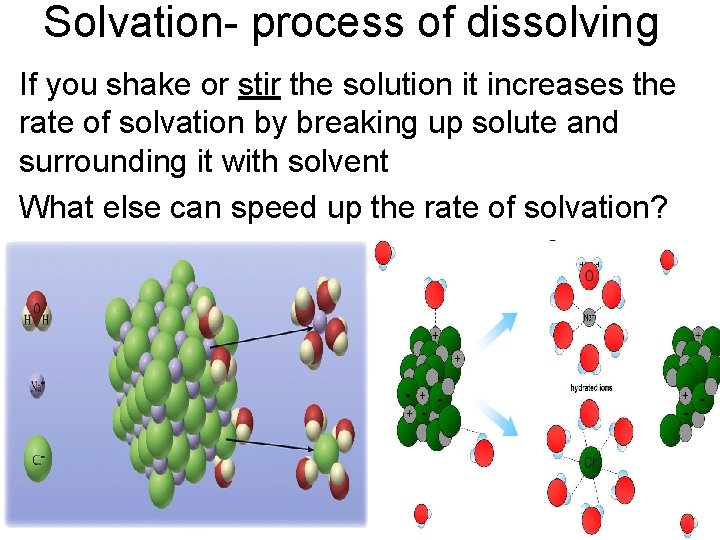
Solvation- process of dissolving If you shake or stir the solution it increases the rate of solvation by breaking up solute and surrounding it with solvent What else can speed up the rate of solvation?

“Like Dissolves Like” Oil on your clothes?
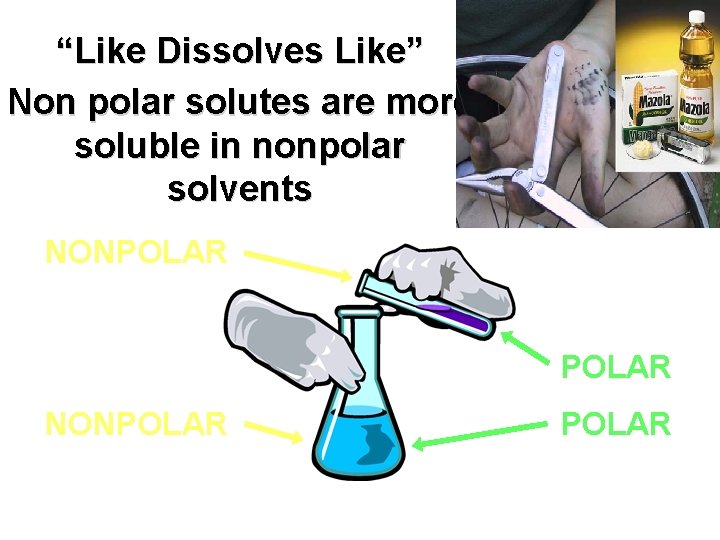
“Like Dissolves Like” Non polar solutes are more soluble in nonpolar solvents NONPOLAR
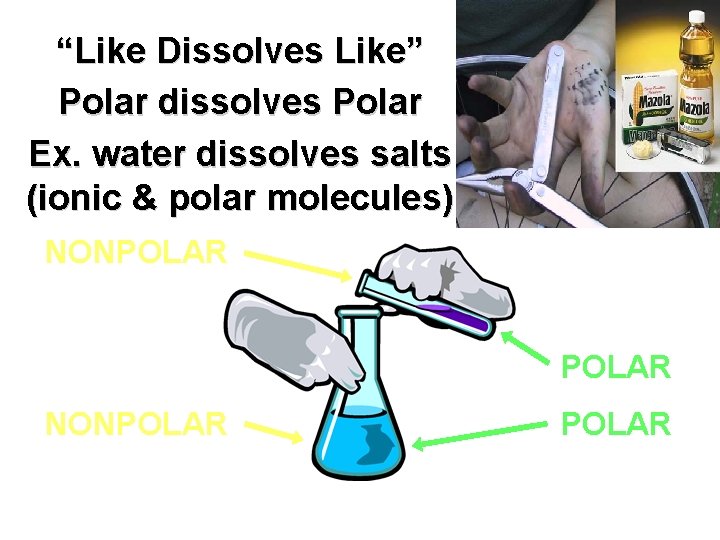
“Like Dissolves Like” Polar dissolves Polar Ex. water dissolves salts (ionic & polar molecules) NONPOLAR
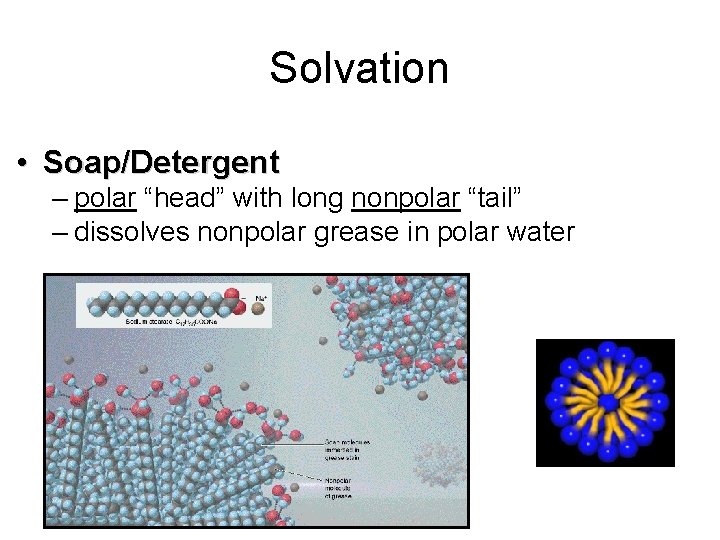
Solvation • Soap/Detergent – polar “head” with long nonpolar “tail” – dissolves nonpolar grease in polar water
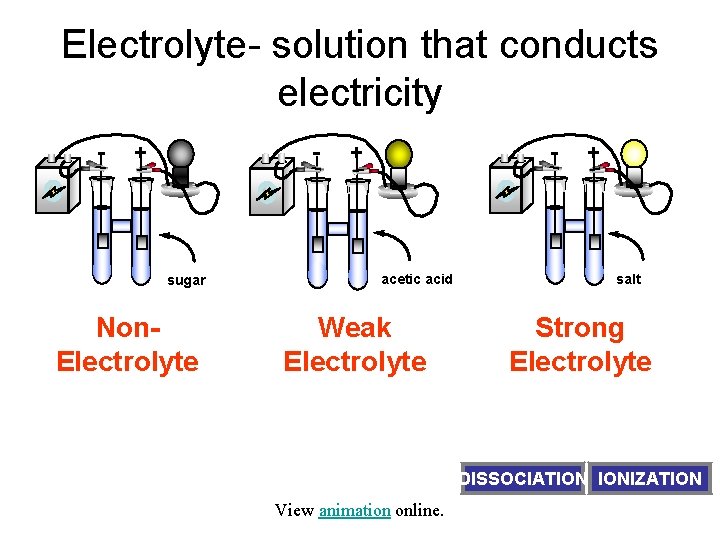
Electrolyte- solution that conducts electricity - - + sugar - + acetic acid + salt Non. Electrolyte Weak Electrolyte Strong Electrolyte solute exists as molecules only solute exists as ions and molecules solute exists as ions only View animation online. DISSOCIATION IONIZATION
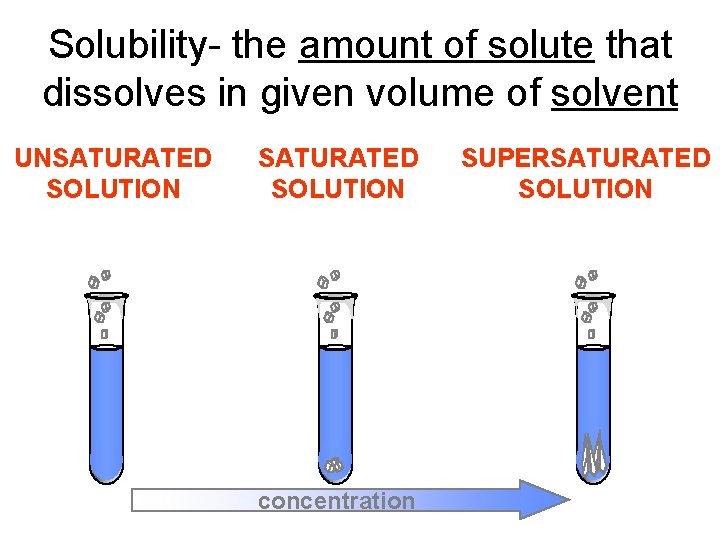
Solubility- the amount of solute that dissolves in given volume of solvent UNSATURATED SOLUTION more solute dissolves SATURATED SOLUTION no more solute dissolves concentration SUPERSATURATED SOLUTION becomes unstable, crystals form

Solubility- the amount of solute that dissolves in given volume of solvent UNSATURATED SUPERSATURATED (dilute) SOLUTION Increase temp. to Small amt. of (no more solute dissolved dissolves) solute concentration

Solubility • Solubility – maximum grams of solute that will dissolve in 100 g of solvent at a given temperature – varies with temp – based on a saturated solution

Definitions • Soluble- any substance that dissolves in something else • Insoluble- Any substance that does not dissolve

Definitions • Miscible – one liquid dissolves in another liquid (ex. 2 polar liquids will be miscible) • Immiscible – one liquid does not dissolve in another liquid (ex. oil and water) don’t mix b/c oil nonpolar and water polar

Definitions • Alloy one metal dissolved in another
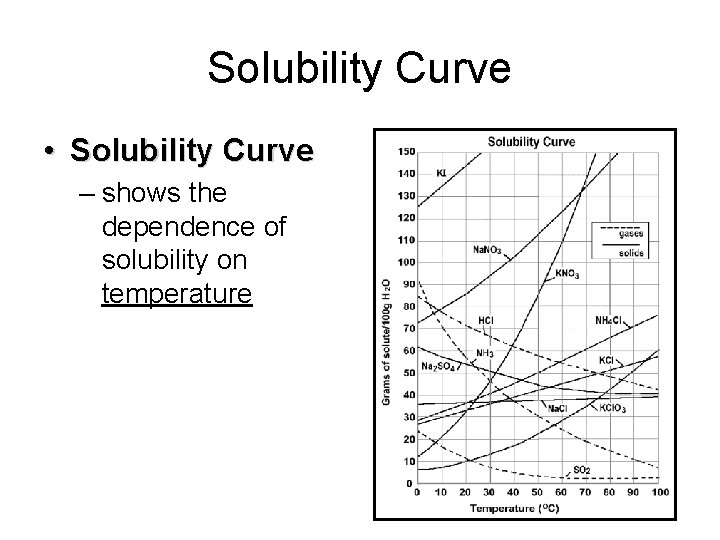
Solubility Curve • Solubility Curve – shows the dependence of solubility on temperature
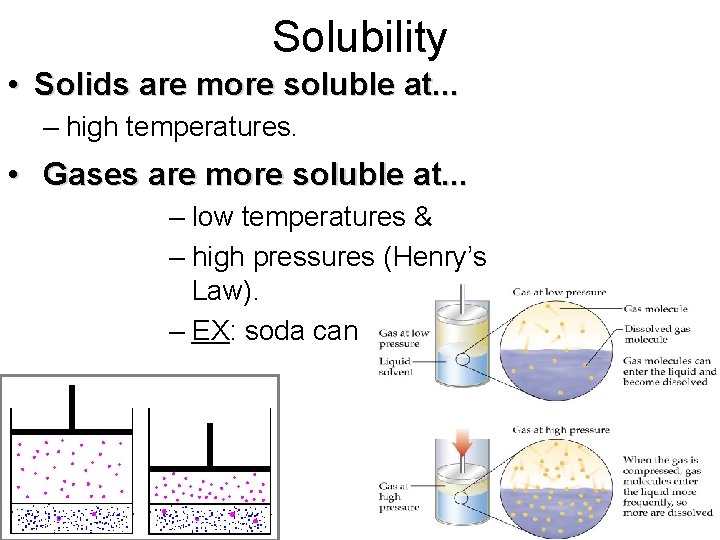
Solubility • Solids are more soluble at. . . – high temperatures. • Gases are more soluble at. . . – low temperatures & – high pressures (Henry’s Law). – EX: soda can

Henry’s Law & Soft Drinks • Soft drinks contain “carbonated water” carbon dioxide gas dissolved in water • Gas in liquid is more soluble if pressure above liquid is high (under pressure) • When bottle opened pressure of CO 2 decreases and solubility of CO 2 also decreases, according to Henry’s Law. • Result, bubbles of CO 2 escape from solution.

Solution and Concentration- a measure of how much solute is dissolved in the solution

Solution and Concentration- a measure of how much solute is dissolved in the solution Range from dilute saturated supersaturated
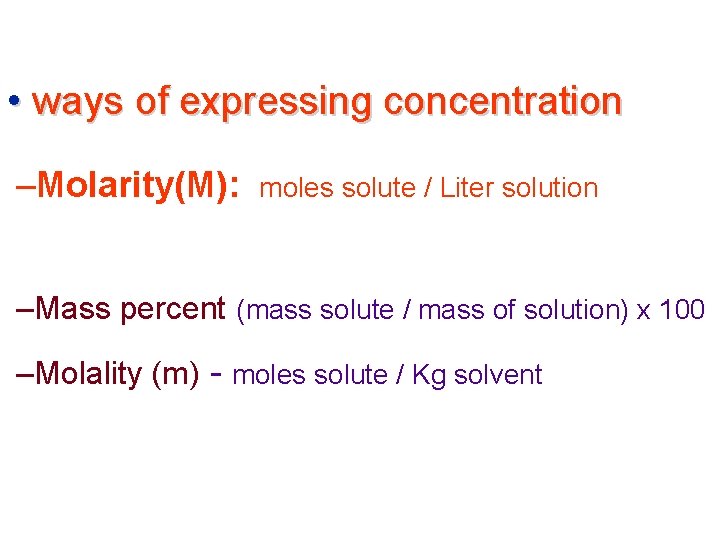
• ways of expressing concentration –Molarity(M): moles solute / Liter solution –Mass percent (mass solute / mass of solution) x 100 –Molality (m) - moles solute / Kg solvent

Molarity(M) = moles solute Liter solution

Calculate the molarity of the following solution 125 ml which contains 2. 50 g Na. Cl

Calculate the molarity of the following solution 125 ml which contains 2. 50 g Na. Cl Molarity = moles of solute Liter of solution
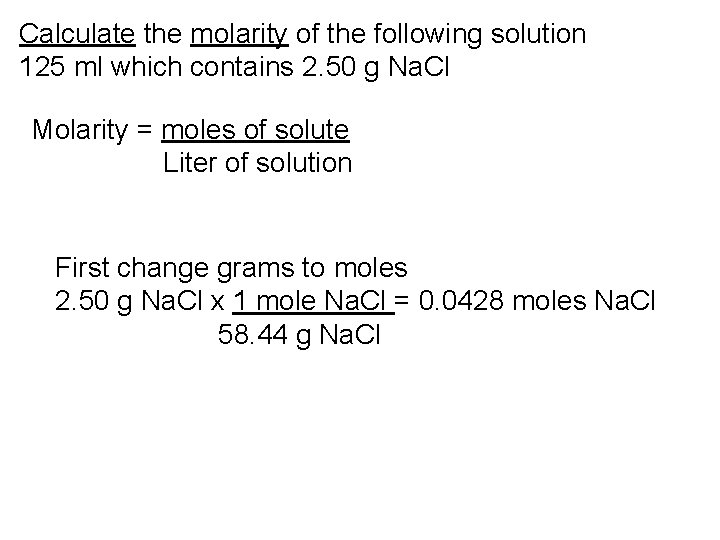
Calculate the molarity of the following solution 125 ml which contains 2. 50 g Na. Cl Molarity = moles of solute Liter of solution First change grams to moles 2. 50 g Na. Cl x 1 mole Na. Cl = 0. 0428 moles Na. Cl 58. 44 g Na. Cl
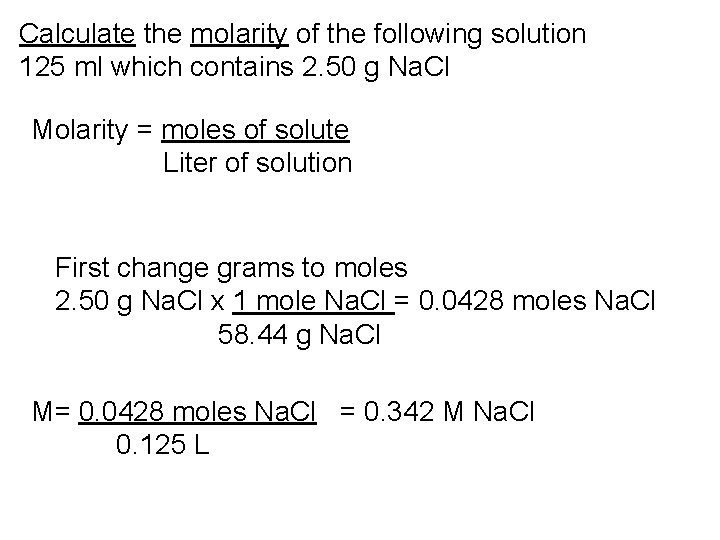
Calculate the molarity of the following solution 125 ml which contains 2. 50 g Na. Cl Molarity = moles of solute Liter of solution First change grams to moles 2. 50 g Na. Cl x 1 mole Na. Cl = 0. 0428 moles Na. Cl 58. 44 g Na. Cl M= 0. 0428 moles Na. Cl = 0. 342 M Na. Cl 0. 125 L

Calculate the mass of the solute in the solution 750 cm cubed of Ca. Cl 2 solution which is 0. 500 M Molarity = moles of solute Liter of solution First change Molarity to moles

Calculate the mass of the solute in the solution 750 cm cubed of Ca. Cl 2 solution which is 0. 500 M (0. 500 moles/1 L) Molarity = moles of solute Liter of solution First change Molarity to moles 0. 500 moles = moles Ca. Cl 2 1 Liter . 750 L

Calculate the mass of the solute in the solution 750 cm cubed of Ca. Cl 2 solution which is 0. 500 M(0. 500 moles/1 L) Molarity = moles of solute Liter of solution First change Molarity to moles 0. 500 moles = moles Ca. Cl 2 1 Liter . 750 L 0. 500 moles x. 750 L = moles Ca. Cl 2 1 Liter

Calculate the mass of the solute in the solution 750 cm cubed of Ca. Cl 2 solution which is 0. 500 M Molarity = moles of solute Liter of solution First change Molarity to moles 0. 500 moles = moles Ca. Cl 2 1 Liter . 750 L 0. 500 moles x. 750 L = moles Ca. Cl 2 1 Liter 0. 500 moles x. 750 L = 0. 375 moles Ca. Cl 2 1 Liter
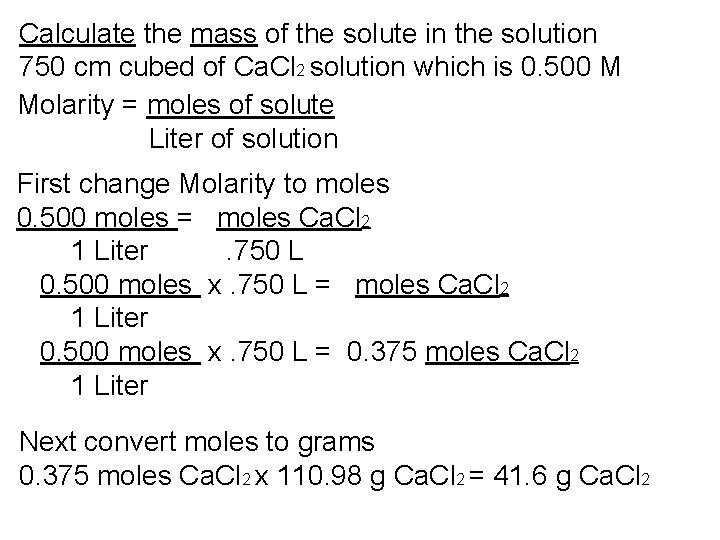
Calculate the mass of the solute in the solution 750 cm cubed of Ca. Cl 2 solution which is 0. 500 M Molarity = moles of solute Liter of solution First change Molarity to moles 0. 500 moles = moles Ca. Cl 2 1 Liter . 750 L 0. 500 moles x. 750 L = moles Ca. Cl 2 1 Liter 0. 500 moles x. 750 L = 0. 375 moles Ca. Cl 2 1 Liter Next convert moles to grams 0. 375 moles Ca. Cl 2 x 110. 98 g Ca. Cl 2 = 41. 6 g Ca. Cl 2

Calculate how many liters of solution can be made from A 2. 00 M solution using 60. 0 g Na. OH Molarity = moles of solute Liter of solution

Calculate how many liters of solution can be made from A 2. 00 M solution using 60. 0 g Na. OH Molarity = moles of solute Liter of solution First change grams of Na. OH to moles 60. 00 g Na. OH x 1 mole Na. OH = 1. 50 moles Na. OH 40. 00 g Na. OH
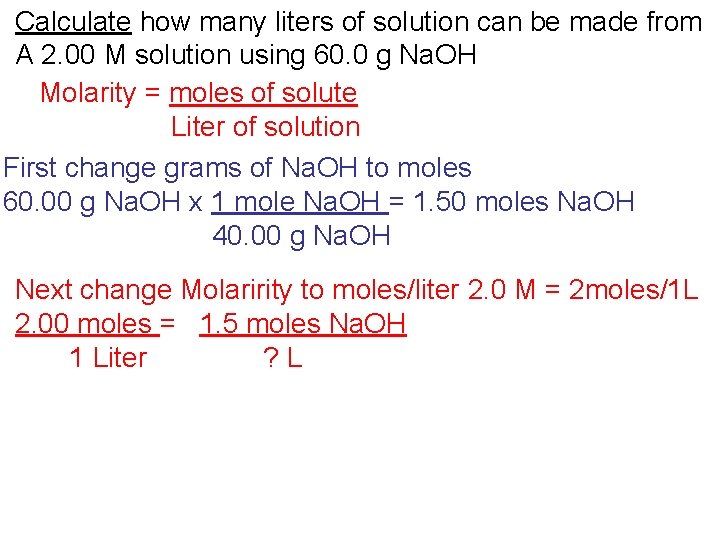
Calculate how many liters of solution can be made from A 2. 00 M solution using 60. 0 g Na. OH Molarity = moles of solute Liter of solution First change grams of Na. OH to moles 60. 00 g Na. OH x 1 mole Na. OH = 1. 50 moles Na. OH 40. 00 g Na. OH Next change Molaririty to moles/liter 2. 0 M = 2 moles/1 L 2. 00 moles = 1. 5 moles Na. OH 1 Liter ? L
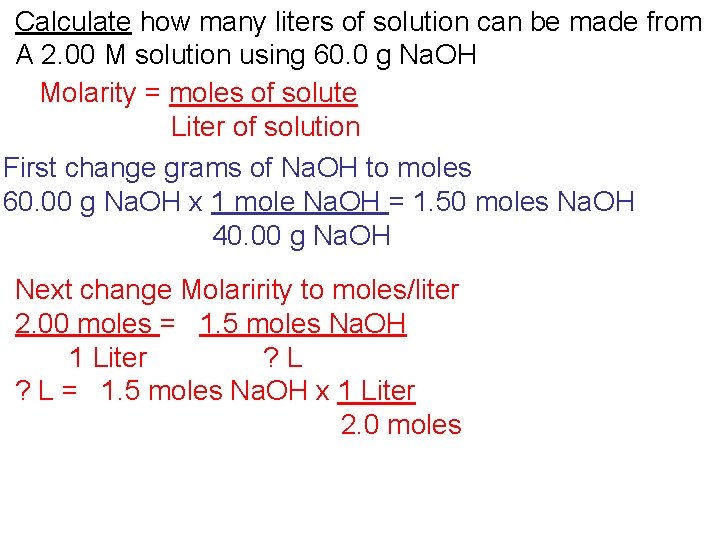
Calculate how many liters of solution can be made from A 2. 00 M solution using 60. 0 g Na. OH Molarity = moles of solute Liter of solution First change grams of Na. OH to moles 60. 00 g Na. OH x 1 mole Na. OH = 1. 50 moles Na. OH 40. 00 g Na. OH Next change Molaririty to moles/liter 2. 00 moles = 1. 5 moles Na. OH 1 Liter ? L = 1. 5 moles Na. OH x 1 Liter 2. 0 moles
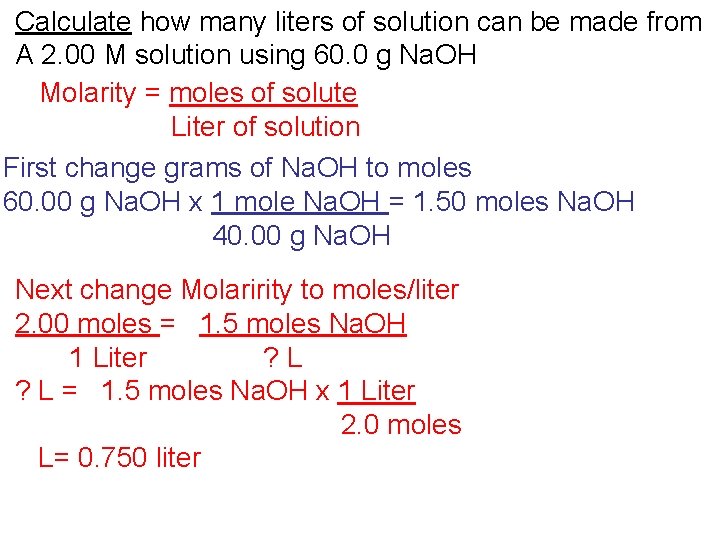
Calculate how many liters of solution can be made from A 2. 00 M solution using 60. 0 g Na. OH Molarity = moles of solute Liter of solution First change grams of Na. OH to moles 60. 00 g Na. OH x 1 mole Na. OH = 1. 50 moles Na. OH 40. 00 g Na. OH Next change Molaririty to moles/liter 2. 00 moles = 1. 5 moles Na. OH 1 Liter ? L = 1. 5 moles Na. OH x 1 Liter 2. 0 moles L= 0. 750 liter
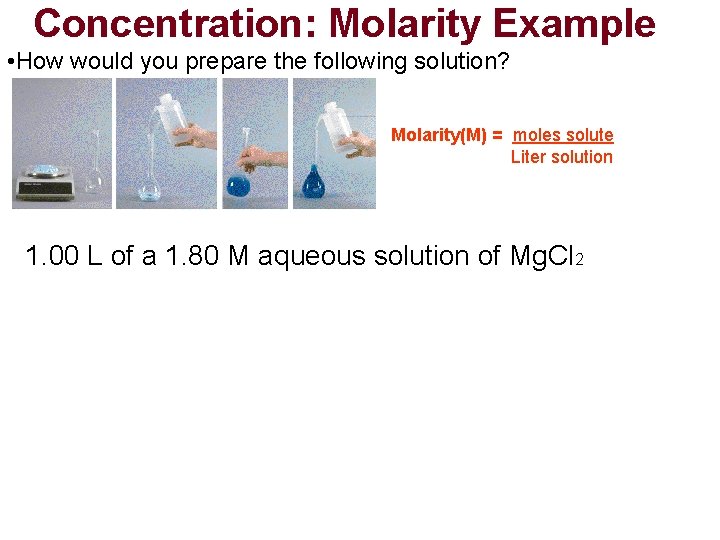
Concentration: Molarity Example • How would you prepare the following solution? Molarity(M) = moles solute Liter solution 1. 00 L of a 1. 80 M aqueous solution of Mg. Cl 2
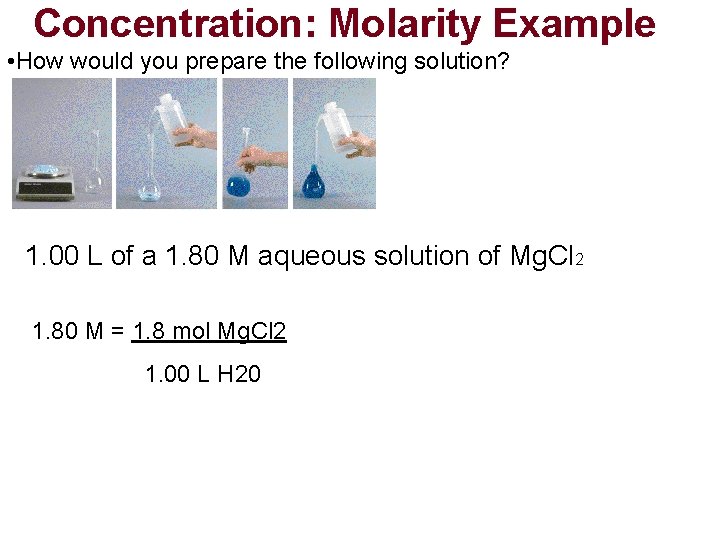
Concentration: Molarity Example • How would you prepare the following solution? 1. 00 L of a 1. 80 M aqueous solution of Mg. Cl 2 1. 80 M = 1. 8 mol Mg. Cl 2 1. 00 L H 20
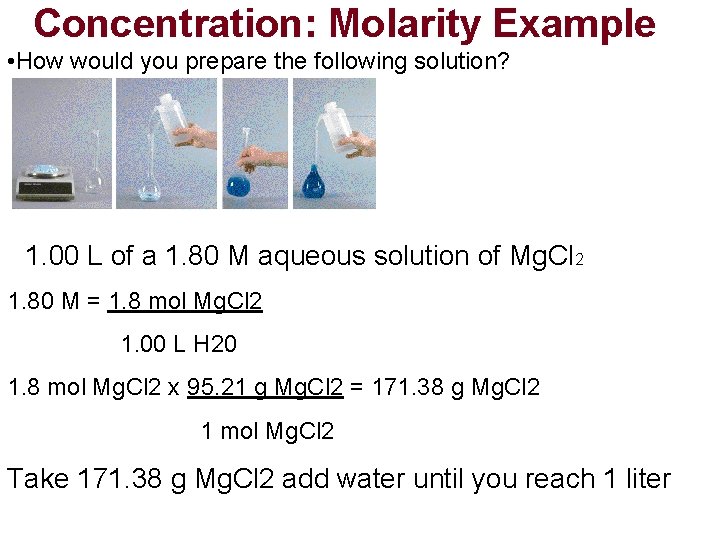
Concentration: Molarity Example • How would you prepare the following solution? 1. 00 L of a 1. 80 M aqueous solution of Mg. Cl 2 1. 80 M = 1. 8 mol Mg. Cl 2 1. 00 L H 20 1. 8 mol Mg. Cl 2 x 95. 21 g Mg. Cl 2 = 171. 38 g Mg. Cl 2 1 mol Mg. Cl 2 Take 171. 38 g Mg. Cl 2 add water until you reach 1 liter
Concentration: Molarity Example • How would you prepare the following solution? 1 L of a 2. 05 M aqueous solution of Mg. Cl 2 2. 05 M = 2. 05 mol Mg. Cl 2 1. 0 L H 20 2. 05 mol Mg. Cl 2 x 95. 21 g Mg. Cl 2 = 195. 18 g Mg. Cl 2 1 mol Mg. Cl 2 Take 195 g Mg. Cl 2 add water until you reach 1 liter
Concentration: Molarity Example 0. 435 g of KMn. O 4 in 250. m. L of solution, what is the molarity of KMn. O 4? Molarity(M) = moles solute Liter solution
Concentration: Molarity Example 0. 435 g of KMn. O 4 in 250. m. L of solution, what is the molarity of KMn. O 4? first step convert the mass of material to moles. 0. 435 g KMn. O 4 • 1 mol KMn. O 4 = 0. 00275 mol KMn. O 4 158. 0 g KMn. O 4
Concentration: Molarity Example 0. 435 g of KMn. O 4 in 250. m. L of solution, what is the molarity of KMn. O 4? 0. 435 g KMn. O 4 • 1 mol KMn. O 4 = 0. 00275 mol KMn. O 4 158. 0 g KMn. O 4 Now convert 250 ml to L 250 m. L = 0. 250 L
Concentration: Molarity Example 0. 435 g of KMn. O 4 in 250. m. L of solution, what is the molarity of KMn. O 4? 0. 435 g KMn. O 4 • 1 mol KMn. O 4 = 0. 00275 mol KMn. O 4 158. 0 g KMn. O 4 Molarity KMn. O 4 = 0. 00275 mol KMn. O 4 = 0. 0110 M 0. 250 L solution
Concentration: Molarity Example 0. 435 g of KMn. O 4 in 250. m. L of solution, what is the molarity of KMn. O 4? As is almost always the case, the first step is to convert the mass of material to moles. 0. 435 g KMn. O 4 • 1 mol KMn. O 4 = 0. 00275 mol KMn. O 4 158. 0 g KMn. O 4 Now that the number of moles of substance is known, this can be combined with the volume of solution — which must be in liters — to give the molarity. Because 250. m. L is equivalent to 0. 250 L. Molarity KMn. O 4 = 0. 00275 mol KMn. O 4 = 0. 0110 M 0. 250 L solution
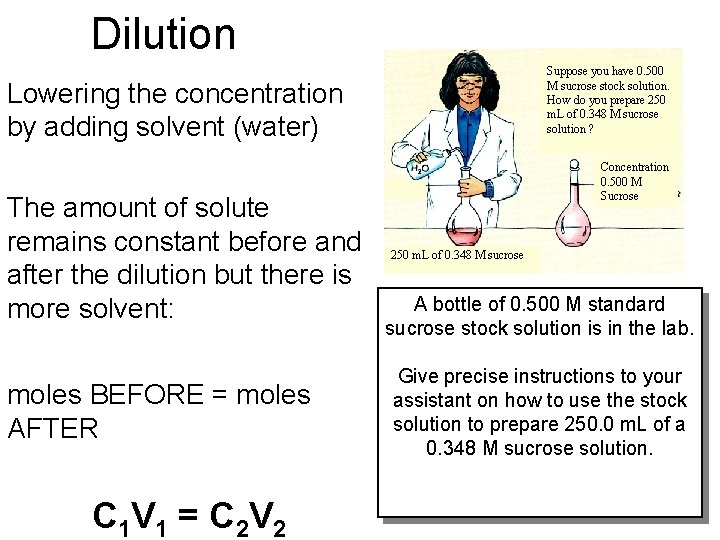
Dilution Suppose you have 0. 500 M sucrose stock solution. How do you prepare 250 m. L of 0. 348 M sucrose solution ? Lowering the concentration by adding solvent (water) The amount of solute remains constant before and after the dilution but there is more solvent: moles BEFORE = moles AFTER C 1 V 1 = C 2 V 2 Concentration 0. 500 M Sucrose 250 m. L of 0. 348 M sucrose A bottle of 0. 500 M standard sucrose stock solution is in the lab. Give precise instructions to your assistant on how to use the stock solution to prepare 250. 0 m. L of a 0. 348 M sucrose solution.
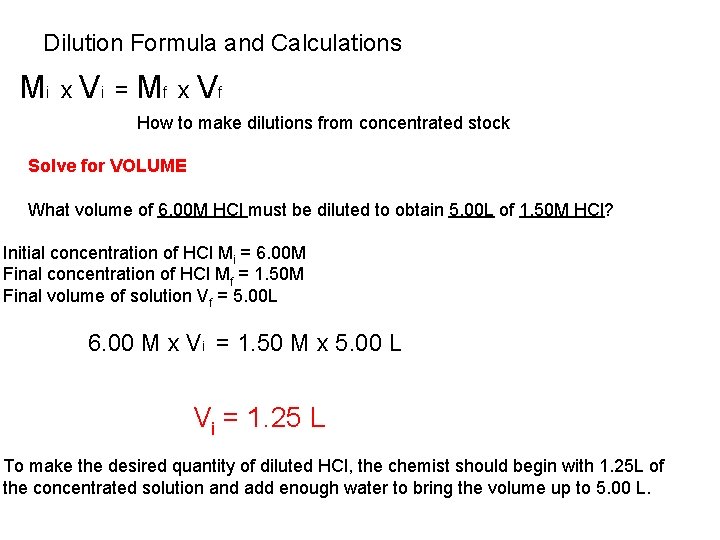
Dilution Formula and Calculations Mi x Vi = Mf x Vf How to make dilutions from concentrated stock Solve for VOLUME What volume of 6. 00 M HCl must be diluted to obtain 5. 00 L of 1. 50 M HCl? Initial concentration of HCl Mi = 6. 00 M Final concentration of HCl Mf = 1. 50 M Final volume of solution Vf = 5. 00 L 6. 00 M x Vi = 1. 50 M x 5. 00 L Vi = 1. 25 L To make the desired quantity of diluted HCl, the chemist should begin with 1. 25 L of the concentrated solution and add enough water to bring the volume up to 5. 00 L.
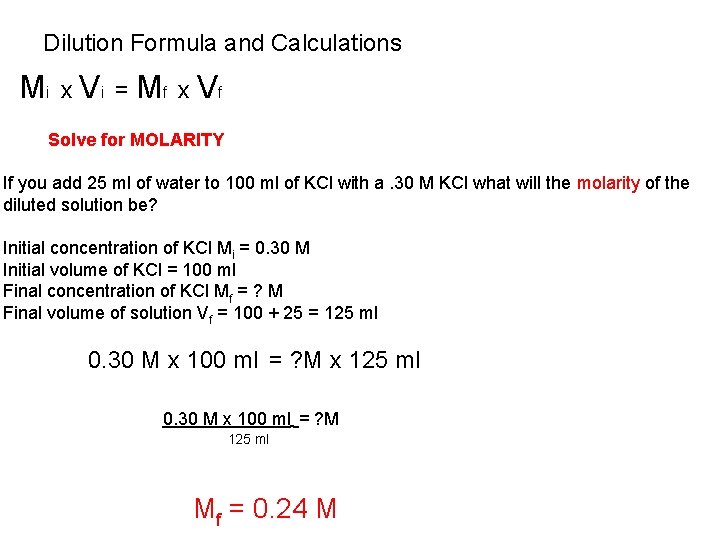
Dilution Formula and Calculations i x i = M V Mf x Vf Solve for MOLARITY If you add 25 ml of water to 100 ml of KCl with a. 30 M KCl what will the molarity of the diluted solution be? Initial concentration of KCl Mi = 0. 30 M Initial volume of KCl = 100 ml Final concentration of KCl Mf = ? M Final volume of solution Vf = 100 + 25 = 125 ml 0. 30 M x 100 ml = ? M x 125 ml 0. 30 M x 100 ml = ? M 125 ml Mf = 0. 24 M

Factors Affecting Solubility 1. Nature of Solute / Solvent. 2. Temperature 3. Pressure Factor (gas) 4. Size of Particle 5. Stirring mixture
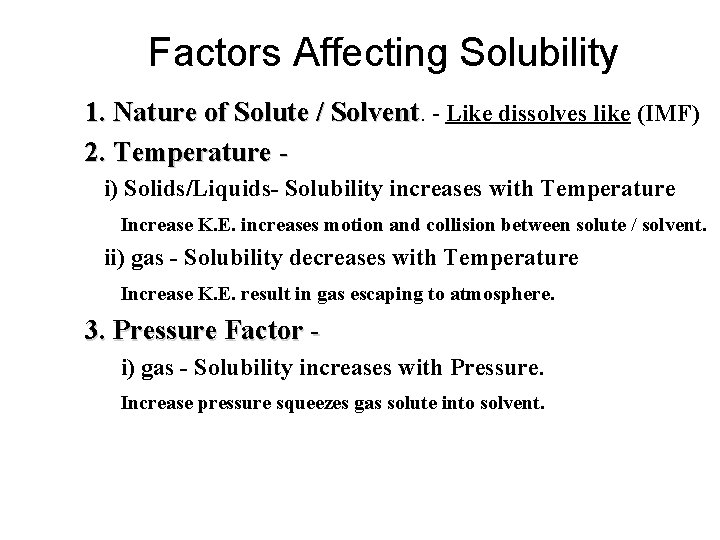
Factors Affecting Solubility 1. Nature of Solute / Solvent. - Like dissolves like (IMF) 2. Temperature i) Solids/Liquids- Solubility increases with Temperature Increase K. E. increases motion and collision between solute / solvent. ii) gas - Solubility decreases with Temperature Increase K. E. result in gas escaping to atmosphere. 3. Pressure Factor i) gas - Solubility increases with Pressure. Increase pressure squeezes gas solute into solvent.
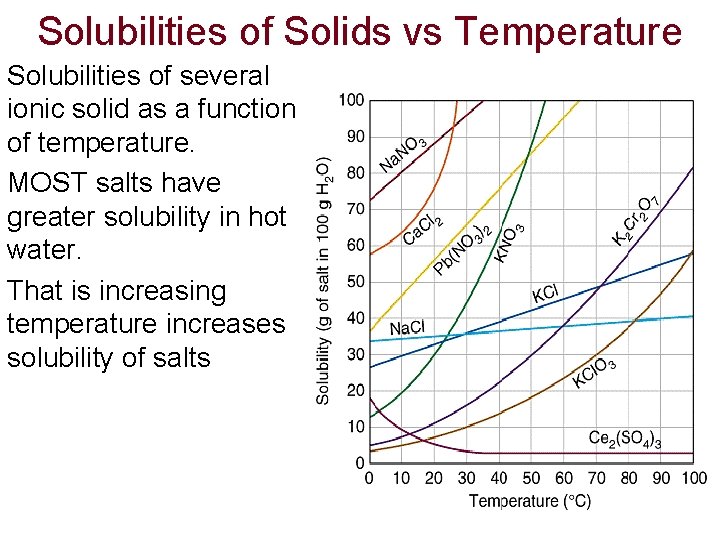
Solubilities of Solids vs Temperature Solubilities of several ionic solid as a function of temperature. MOST salts have greater solubility in hot water. That is increasing temperature increases solubility of salts

Colligative Properties are properties of a liquid that change when a solute is added.

Colligative Properties Dissolving solute in pure liquid will change all physical properties of liquid, Density, Vapor Pressure, Boiling Point, Freezing Point, Osmotic Pressure

Colligative Properties The magnitude of the change depends on the number of solute particles in the solution, NOT on number the identity of the solute particles. identity
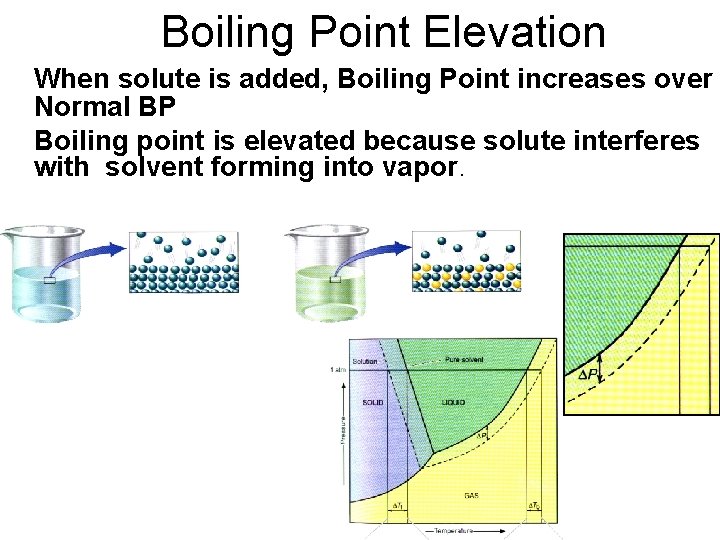
Boiling Point Elevation When solute is added, Boiling Point increases over Normal BP Boiling point is elevated because solute interferes with solvent forming into vapor.
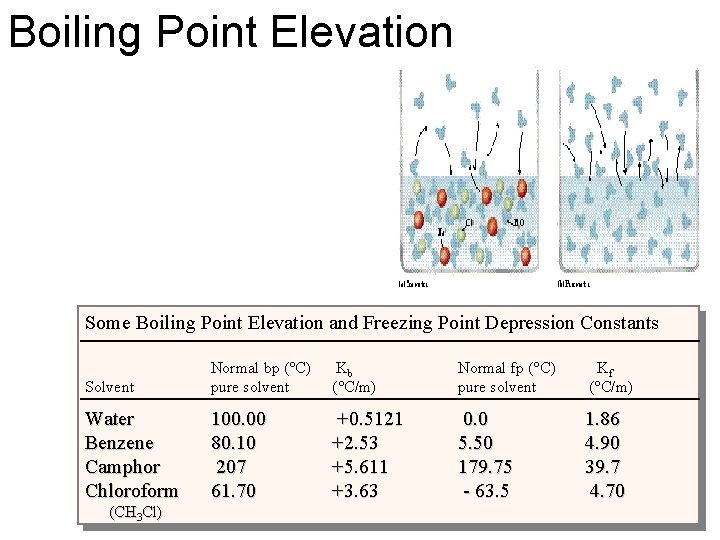
Boiling Point Elevation Some Boiling Point Elevation and Freezing Point Depression Constants Solvent Normal bp (°C) pure solvent Kb (°C/m) Normal fp (°C) pure solvent Kf (°C/m) Water Benzene Camphor Chloroform 100. 00 80. 10 207 61. 70 +0. 5121 +2. 53 +5. 611 +3. 63 0. 0 5. 50 179. 75 - 63. 5 1. 86 4. 90 39. 7 4. 70 (CH 3 Cl)
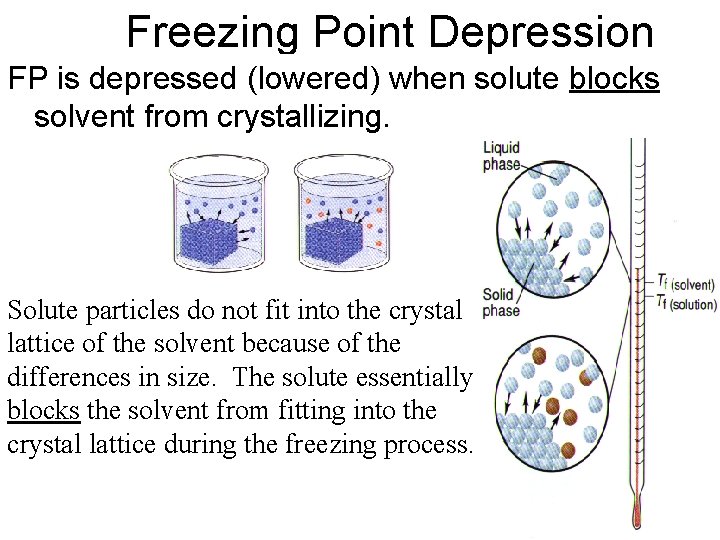
Freezing Point Depression FP is depressed (lowered) when solute blocks solvent from crystallizing. Solute particles do not fit into the crystal lattice of the solvent because of the differences in size. The solute essentially blocks the solvent from fitting into the crystal lattice during the freezing process.
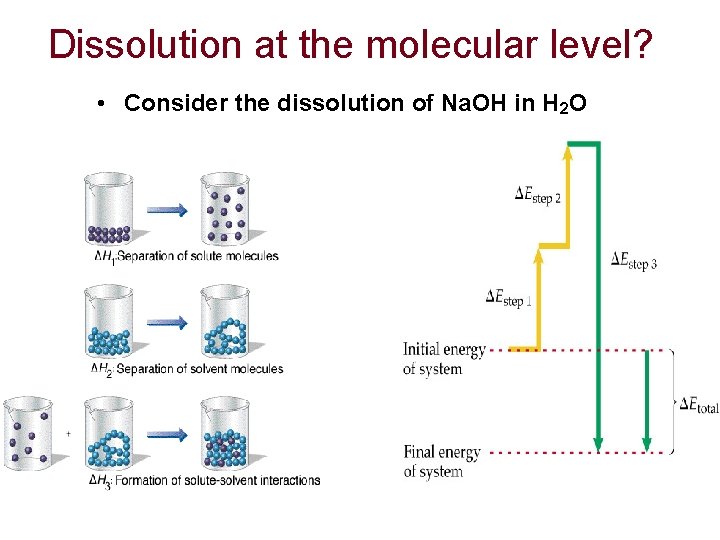
Dissolution at the molecular level? • Consider the dissolution of Na. OH in H 2 O
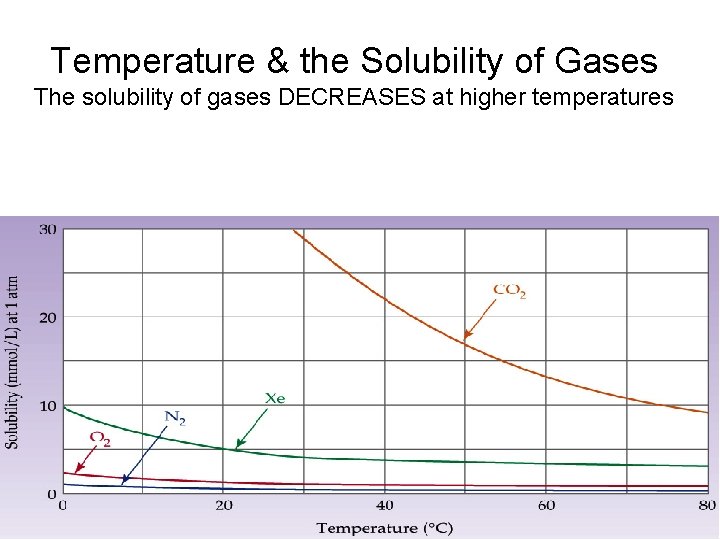
Temperature & the Solubility of Gases The solubility of gases DECREASES at higher temperatures
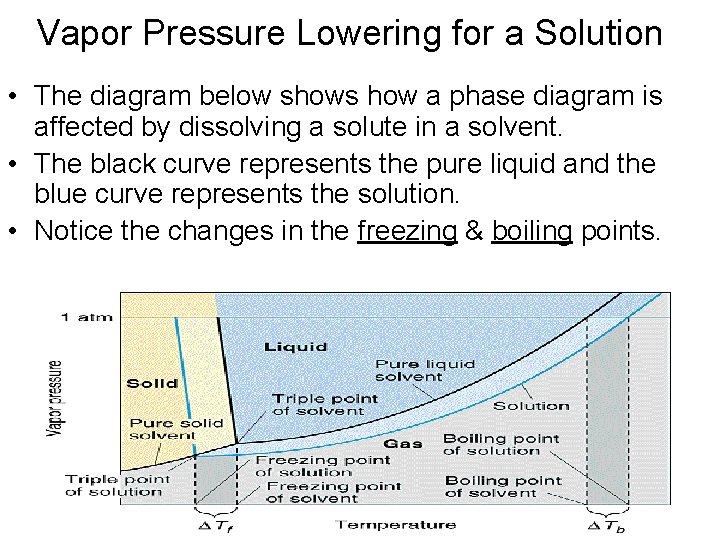
Vapor Pressure Lowering for a Solution • The diagram below shows how a phase diagram is affected by dissolving a solute in a solvent. • The black curve represents the pure liquid and the blue curve represents the solution. • Notice the changes in the freezing & boiling points.
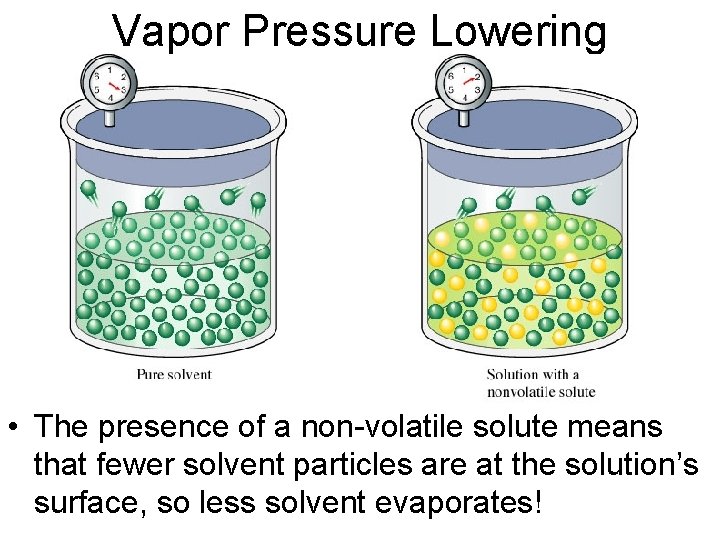
Vapor Pressure Lowering • The presence of a non-volatile solute means that fewer solvent particles are at the solution’s surface, so less solvent evaporates!
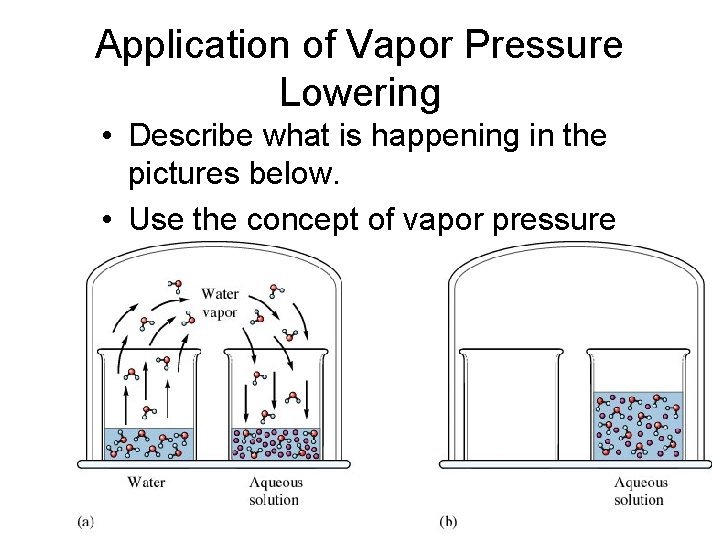
Application of Vapor Pressure Lowering • Describe what is happening in the pictures below. • Use the concept of vapor pressure lowering to explain this phenomenon.

Osmotic pressure • Osmosis is the spontaneous movement of water across a semi-permeable membrane from an area of low solute concentration to an area of high solute concentration • Osmotic Pressure - The Pressure that must be applied to stop osmosis P = i CRT where P = osmotic pressure i = van’t Hoff factor C = molarity R = ideal gas constant T = Kelvin temperature
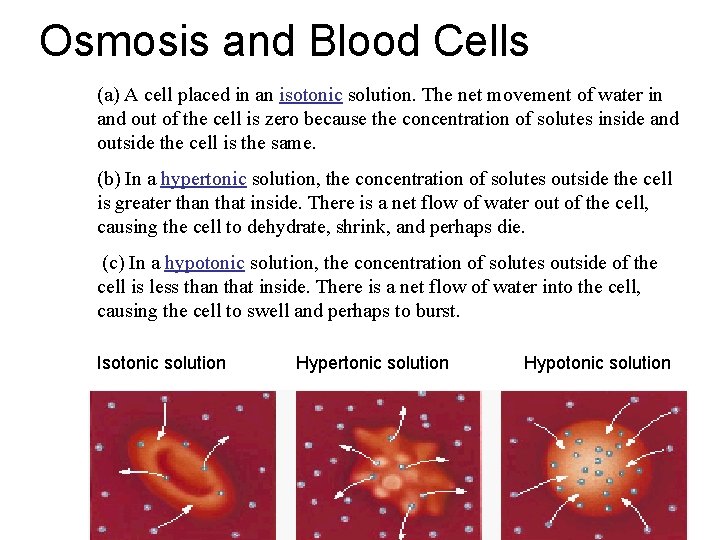
Osmosis and Blood Cells (a) A cell placed in an isotonic solution. The net movement of water in and out of the cell is zero because the concentration of solutes inside and outside the cell is the same. (b) In a hypertonic solution, the concentration of solutes outside the cell is greater than that inside. There is a net flow of water out of the cell, causing the cell to dehydrate, shrink, and perhaps die. (c) In a hypotonic solution, the concentration of solutes outside of the cell is less than that inside. There is a net flow of water into the cell, causing the cell to swell and perhaps to burst. Isotonic solution Hypertonic solution Hypotonic solution
- Slides: 65