Solutions Definitions Solution Homogeneous mixture of two or



















- Slides: 30

Solutions

Definitions • Solution – Homogeneous mixture of two or more substances • Solute – Substance that is dissolved • Solvent – Substance that dissolves the solute • Aqueous Solution – water is the solvent Example: Na. Cl(aq) = Na. Cl + H 2 O (solute) (solvent)

Concentration • Concentration - Quantity of solute in a given measure (volume or mass) of solution • Concentration can be expressed as: – Molarity (M) – Percent Composition/Percent Mass – Parts per million (ppm)

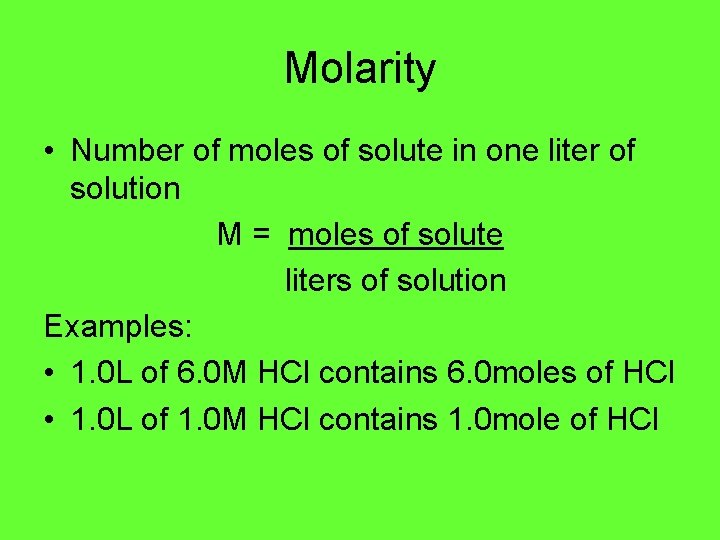
Molarity • Number of moles of solute in one liter of solution M = moles of solute liters of solution Examples: • 1. 0 L of 6. 0 M HCl contains 6. 0 moles of HCl • 1. 0 L of 1. 0 M HCl contains 1. 0 mole of HCl

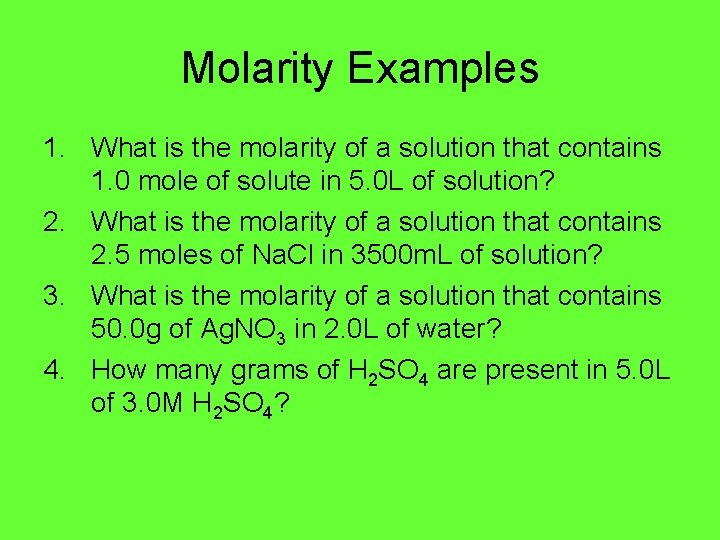
Molarity Examples 1. What is the molarity of a solution that contains 1. 0 mole of solute in 5. 0 L of solution? 2. What is the molarity of a solution that contains 2. 5 moles of Na. Cl in 3500 m. L of solution? 3. What is the molarity of a solution that contains 50. 0 g of Ag. NO 3 in 2. 0 L of water? 4. How many grams of H 2 SO 4 are present in 5. 0 L of 3. 0 M H 2 SO 4?

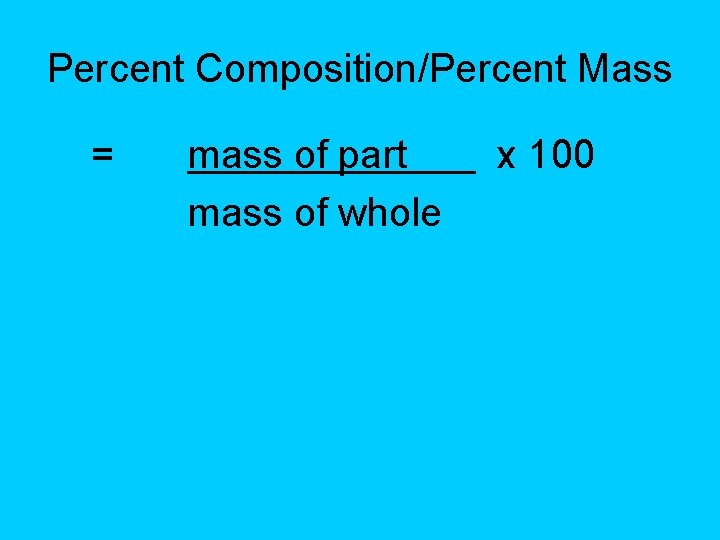
Percent Composition/Percent Mass = mass of part mass of whole x 100

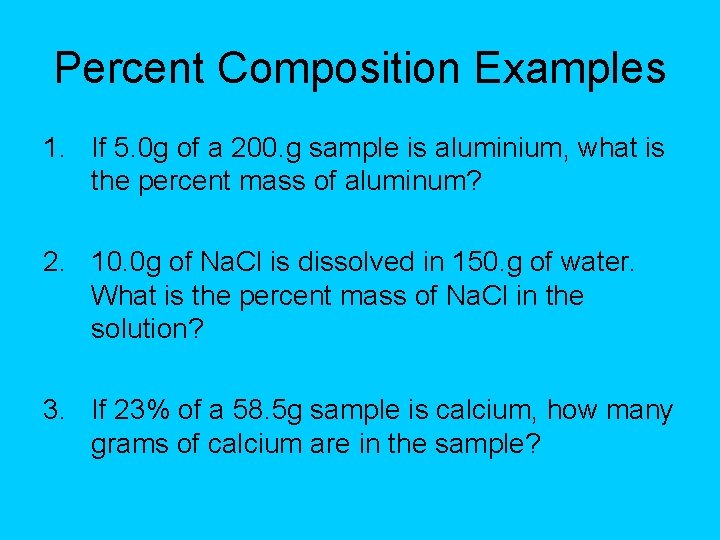
Percent Composition Examples 1. If 5. 0 g of a 200. g sample is aluminium, what is the percent mass of aluminum? 2. 10. 0 g of Na. Cl is dissolved in 150. g of water. What is the percent mass of Na. Cl in the solution? 3. If 23% of a 58. 5 g sample is calcium, how many grams of calcium are in the sample?

Parts per million (ppm) = grams of solute x 1, 000 grams of solution

ppm Examples 1. Calculate the concentration (in ppm) if 10. 0 g of Na. Cl is dissolved in 750 g of solution. 2. Calculate the concentration (in ppm) if 1. 25 g of Cu. SO 4 is dissolved in 550 g of water. 3. How much Fe is in a 5000. g solution, if it has an iron concentration of 500. ppm?

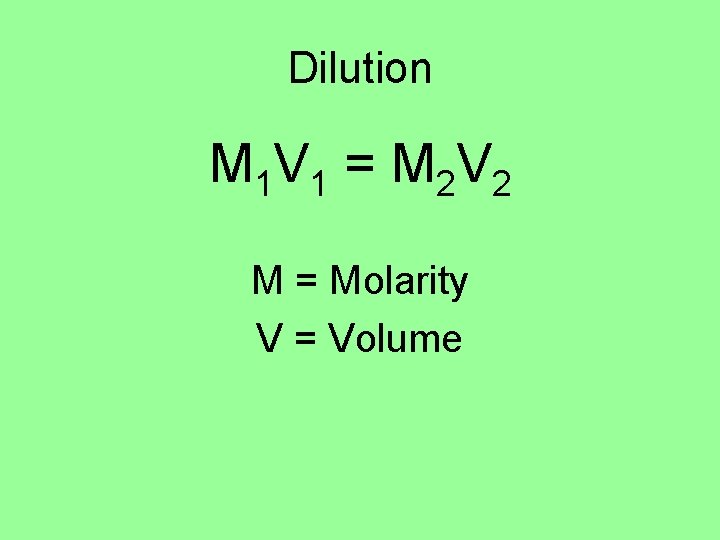
Dilution M 1 V 1 = M 2 V 2 M = Molarity V = Volume

Dilution Examples 1. How many milliliters of 12 M HCl must be added to water to make a 300. 0 m. L solution of 6. 0 M HCl? 2. How many milliliters of 18 M H 2 SO 4 must be used to make a 1. 0 L sample of 1. 0 M H 2 SO 4? 3. 15 m. L of 3. 0 M Na. OH are diluted to a volume of 500. m. L. What is the resulting concentration of the diluted solution?

Solubility Depends on: 1. The nature of the solute and solvent – “Like dissolves Like” • Polar solvents dissolve polar and ionic solutes • Nonpolar solvents dissolve nonpolar solutes

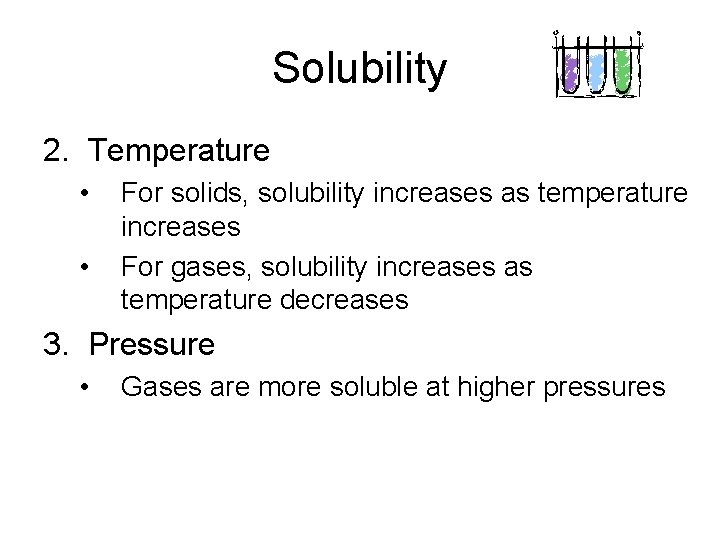
Solubility 2. Temperature • • For solids, solubility increases as temperature increases For gases, solubility increases as temperature decreases 3. Pressure • Gases are more soluble at higher pressures

Factors that affect the rate of dissolving 1. Size of the particles • More surface area (smaller pieces) = faster 2. Stirring 3. Amount of solute already dissolved 4. Temperature

Saturated Solutions • Contain the exact amount of solute that can be dissolved at a certain temperature and pressure • Solute is added under constant conditions until no more will dissolve • If additional solute is added it will not dissolve

Unsaturated Solutions • Contain less solute then can be dissolved at a certain temperature and pressure • If additional solute is added it will dissolve

Supersaturated Solutions • Contains more solute then should be dissolved (more than the saturated solution) • A saturated solution is made at a high temperature and allowed to gradually cool • Very rare, very unstable • If additional solute is added the “extra” solute will fall out of solution (re-crystallize)

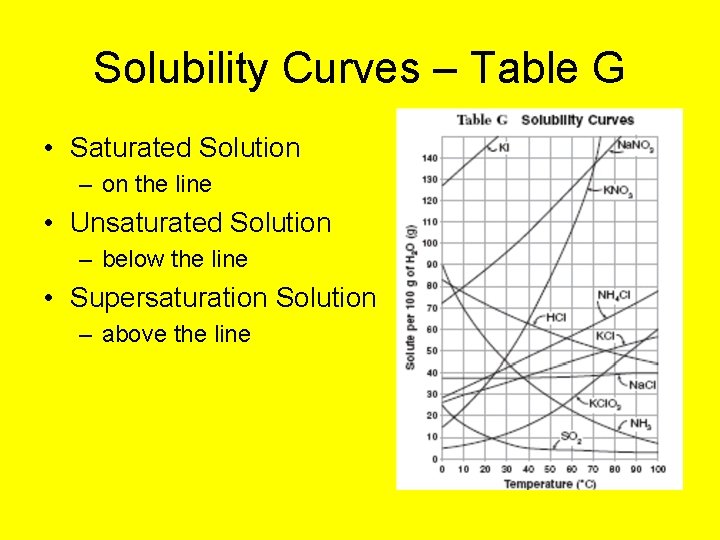
Solubility Curves – Table G • Saturated Solution – on the line • Unsaturated Solution – below the line • Supersaturation Solution – above the line

Examples 1. 60 g of NH 4 Cl is dissolved in 100 g of water at 70 o. C. What type of solution is this? 2. How many grams of Na. Cl must be dissolved in 100 g of water at 40 o. C to make a saturated solution?

Examples 3. How many grams of KNO 3 must be dissolved in 200 g of water at 55 o. C to make a saturated solution? 4. A solution contains 25 g of KCl in 100 g of water at 50 o. C. How much additional KCl must be added to make a saturated solution?

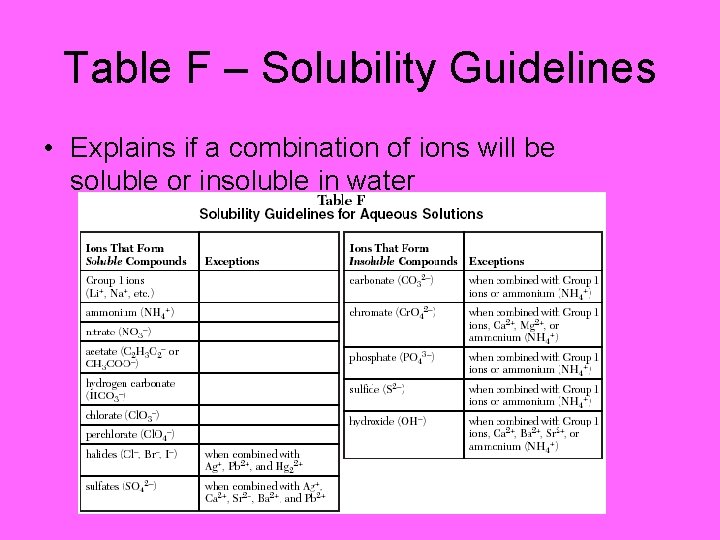
Table F – Solubility Guidelines • Explains if a combination of ions will be soluble or insoluble in water

Table F Examples • 1. 2. 3. 4. 5. Indicate if the following are soluble or insoluble Li. NO 3 Li 3 PO 4 Na. OH Al(OH)3 Mg. CO 3

Colligative Properties • Properties that depend on the number of particles in solution Examples: Boiling Point, Freezing Point, Vapor Pressure

Boiling Point / Freezing Point • The addition of a solute to a solvent causes the – Boiling point to increase – Freezing point to decrease • The great the concentration of particles the greater the effect Example: The addition of antifreeze (ethylene glycol) to your radiator increases the boiling point and decreases the freezing point

Boiling Point • The addition of a solute raises the boiling point of the solvent • One mole of particles raises the boiling point of water by 0. 52 o. C

Freezing Point • The addition of a solute lowers the freezing point of the solvent • One mole of particles lowers the freezing point of water by 1. 86 o. C

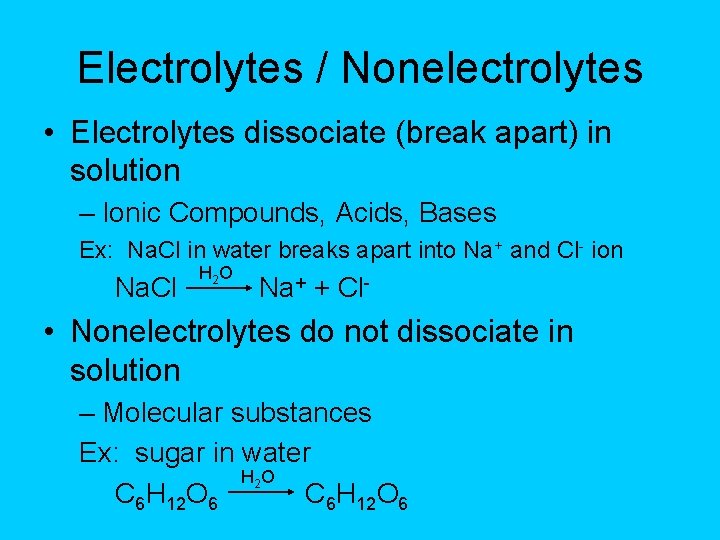
Electrolytes / Nonelectrolytes • Electrolytes dissociate (break apart) in solution – Ionic Compounds, Acids, Bases Ex: Na. Cl in water breaks apart into Na+ and Cl- ion Na. Cl H 2 O Na+ + Cl- • Nonelectrolytes do not dissociate in solution – Molecular substances Ex: sugar in water H 2 O C 6 H 12 O 6

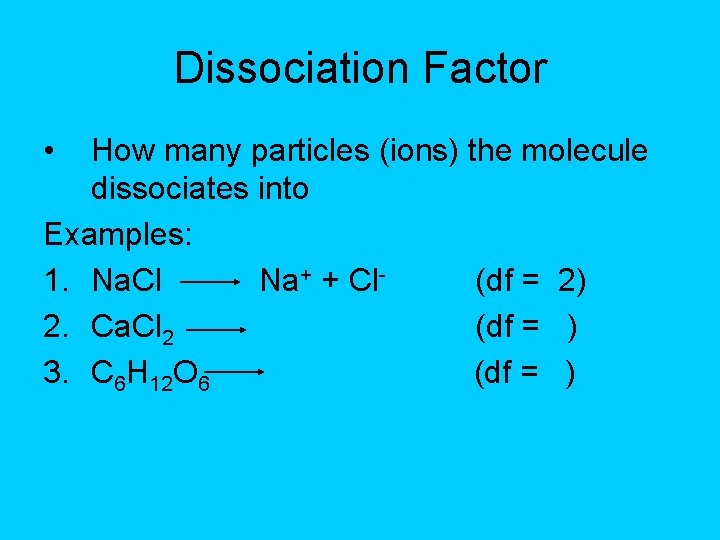
Dissociation Factor • How many particles (ions) the molecule dissociates into Examples: 1. Na. Cl Na+ + Cl(df = 2) 2. Ca. Cl 2 (df = ) 3. C 6 H 12 O 6 (df = )

Examples 1. Equal amounts of which will lower the freezing point of water more? a. Sugar (C 6 H 12 O 6) b. Salt (Na. Cl) 2. Explain why Na. Cl breaks into 2 particles (df = 2), sugar does not break apart (df = 1)

Examples 3. Which would have a lower freezing point, 3. 0 M Ca. Cl 2(aq) or 3. 0 M Na. Cl(aq)? Explain why. 4. Which will have a higher boiling point, 6. 0 M HCl or 3. 0 M HCl? Explain why. 5. Which would have a lower freezing point, 6. 0 M HCl or 3. 0 M HCl? Expain why.