Solutions Definitions Solute substance being dissolved Solvent substance
Solutions
Definitions Solute - substance being dissolved Solvent – substance that is doing the dissolving (usually present in greater amounts) Solution – a mixture of substances that has uniform composition (homogenous mixture)

Solutions: Basic Definitions Soluble – when a substance will dissolve in another substance Ex: salt and water Insoluble – when a substance will not dissolve in another substance Ex: sand & water

Solutions: Basic Definitions Miscible – when two liquids are soluble in each other Ex: alcohol & water Immiscible – when two liquids are not soluble in each other Ex: oil & water Aqueous – a solid dissolved in water
Solutions: Basic Definitions Electrolyte – a solution that separates into charged particles that conducts an electric current Non electrolyte – solution that separates into charged particles that does not conduct an electric current
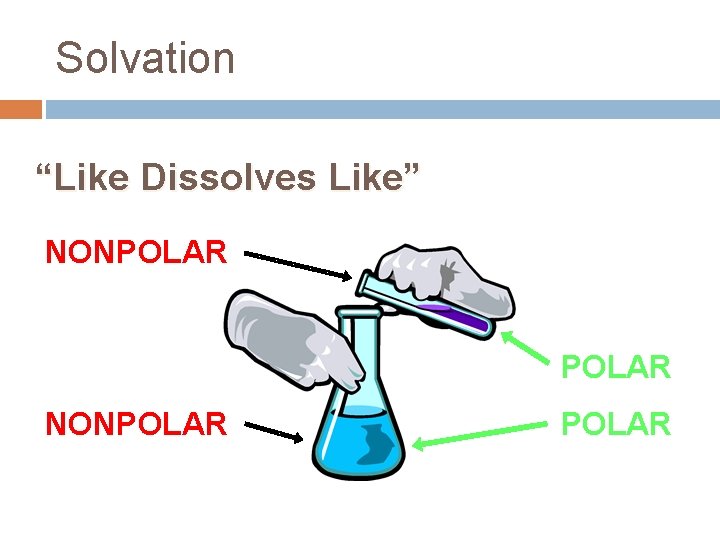
Solvation “Like Dissolves Like” NONPOLAR
Polar vs. nonpolar? Molecules may be polar or non‐polar depending on the types of bonds and the arrangement of the bonds, that is, the shape of the molecule. In general: Ø Polar – unequal distribution of electrons • Ionic Compounds = Polar • EX: Water is the universal solvent for polar compounds Ø Nonpolar – equal distribution of electrons • Covalent Compounds = Nonpolar ***Remember the phrase “LIKE DISSOLVES LIKE”
Increasing the Rate of Solution Agitation If you increase the speed of the particles, you increase the dissolving process Increasing Temperature As you increase the temperature, more particles will collide (high KE), thus increasing the rate of reaction Increasing Surface Area Smaller particles dissolve faster than larger particles due to an increased surface area Which will dissolve faster: Sugar cube vs.

Solubility Maximum grams of solute that will dissolve in 100 g of solvent at a given temperature Varies with temperature Based on a saturated solution
Solutions: Basic Definitions unsaturated solution - If the amount of solute dissolved is less than the maximum that could be dissolved (point below the line) saturated solution - solution which holds the maximum amount of solute per amount of the solution under the given conditions (point on the line) supersaturated solution - solutions that contain more solute than the usual maximum amount and are unstable (point above the line) Which solution allows for more solute to be added?
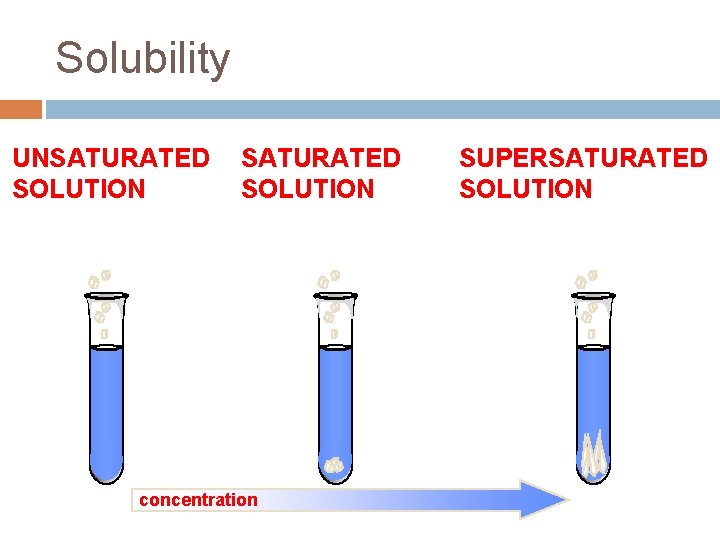
Solubility UNSATURATED SOLUTION more solute dissolves SATURATED SOLUTION no more solute dissolves concentration SUPERSATURATED SOLUTION becomes unstable, crystals form
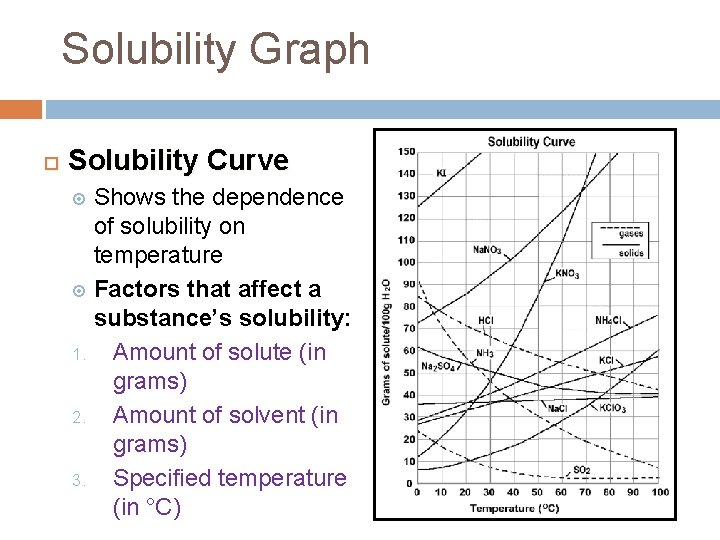
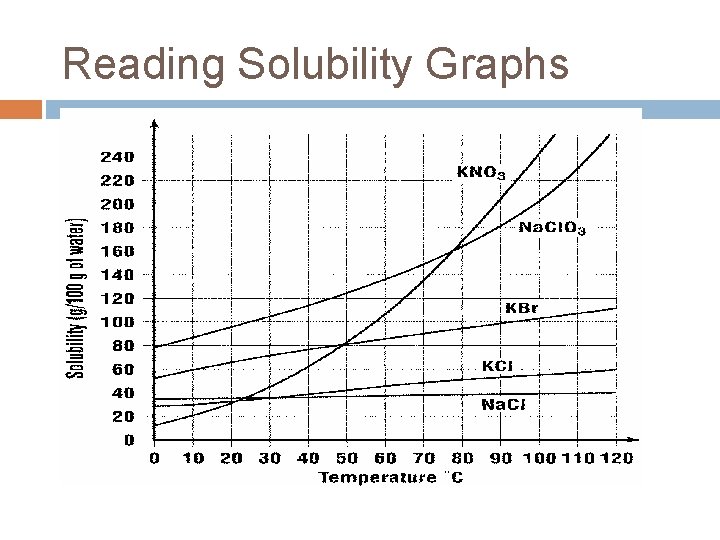
Solubility Graph Solubility Curve Shows the dependence of solubility on temperature Factors that affect a substance’s solubility: 1. Amount of solute (in grams) 2. Amount of solvent (in grams) 3. Specified temperature (in °C)
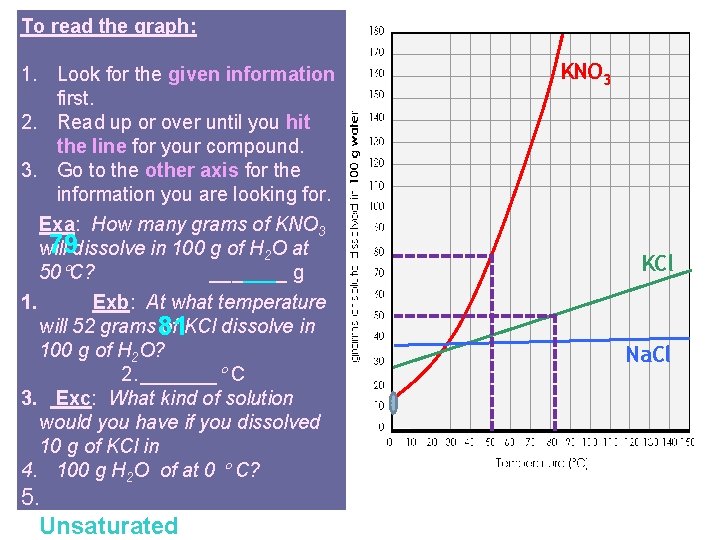
To read the graph: 1. Look for the given information first. 2. Read up or over until you hit the line for your compound. 3. Go to the other axis for the information you are looking for. Exa: How many grams of KNO 3 79 dissolve in 100 g of H 2 O at will 50 C? _______ g 1. Exb: At what temperature will 52 grams 81 of KCl dissolve in 100 g of H 2 O? 2. _______ C 3. Exc: What kind of solution would you have if you dissolved 10 g of KCl in 4. 100 g H 2 O of at 0 C? 5. Unsaturated KNO 3 KCl Na. Cl
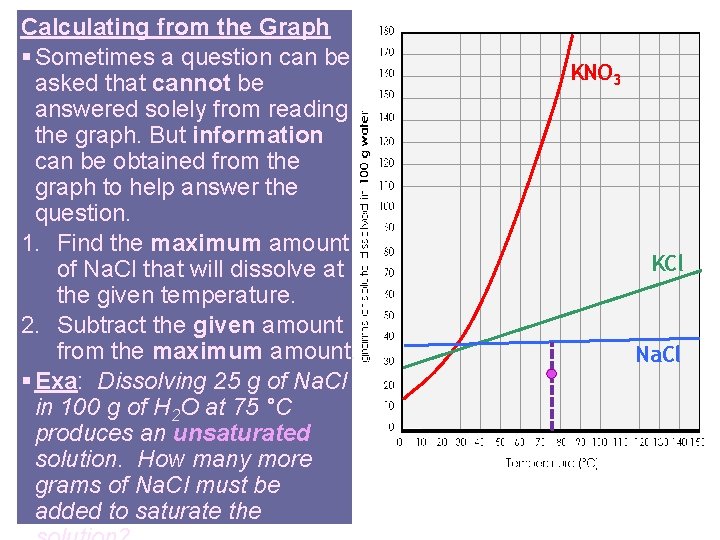
Calculating from the Graph § Sometimes a question can be asked that cannot be answered solely from reading the graph. But information can be obtained from the graph to help answer the question. 1. Find the maximum amount of Na. Cl that will dissolve at the given temperature. 2. Subtract the given amount from the maximum amount. § Exa: Dissolving 25 g of Na. Cl in 100 g of H 2 O at 75 °C produces an unsaturated solution. How many more grams of Na. Cl must be added to saturate the KNO 3 KCl Na. Cl
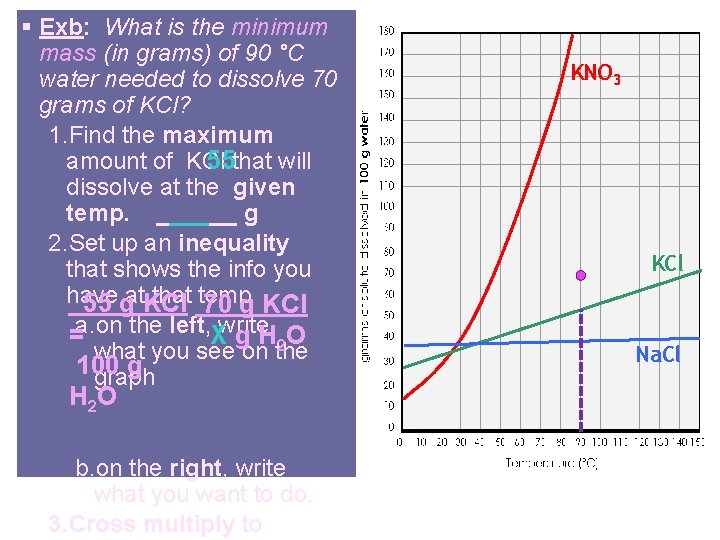
§ Exb: What is the minimum mass (in grams) of 90 °C water needed to dissolve 70 grams of KCl? 1. Find the maximum amount of KCl 55 that will dissolve at the given temp. ______ g 2. Set up an inequality that shows the info you have that temp 55 gat. KCl 70 g KCl =a. on the left, Xwrite g H 2 O what you see on the 100 g graph H 2 O b. on the right, write what you want to do. 3. Cross multiply to KNO 3 KCl Na. Cl
Reading Solubility Graphs
- Slides: 16