Solutions Classification of Matter Pure Substances Elements Compounds

Solutions!
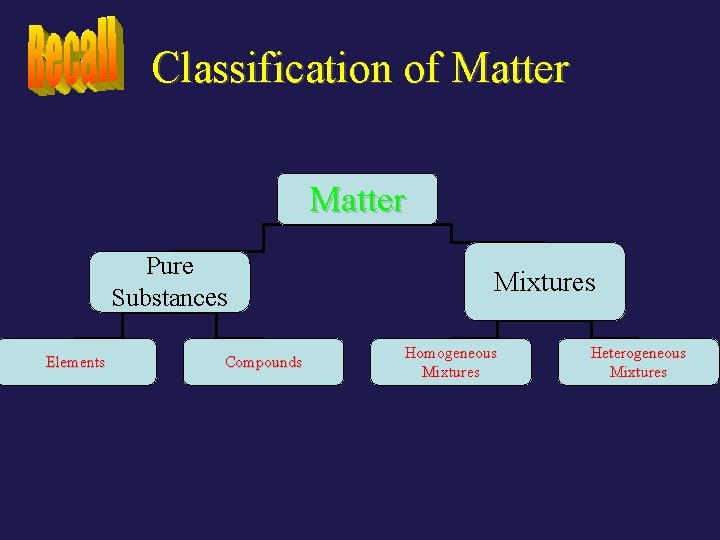
Classification of Matter Pure Substances Elements Compounds Mixtures Homogeneous Mixtures Heterogeneous Mixtures

Heterogeneous Mixtures • See visibly different regions – Granite – Dirt – Cereals – Oil & Vinegar • See a boundary – Ice cube in water

Homogeneous Mixtures • Particles very small – on atomic scale – Can’t see particles – Can’t sort particles – Can’t get trapped by filter – Can’t scatter light • • Particles evenly distributed Particles do not separate
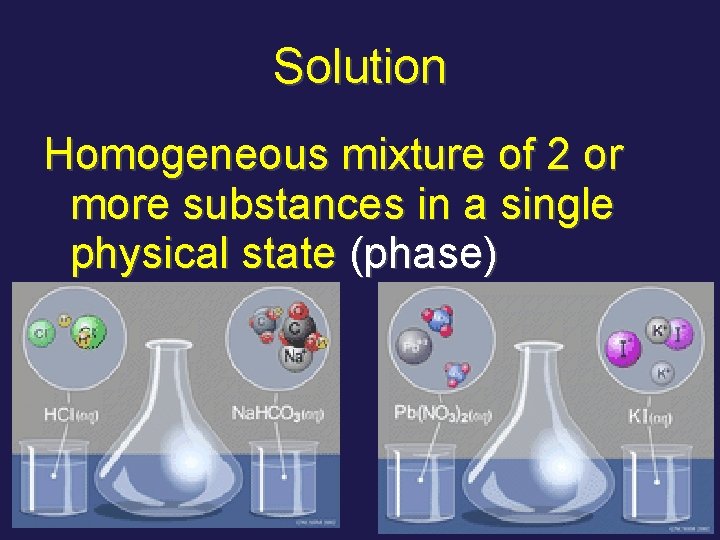
Solution Homogeneous mixture of 2 or more substances in a single physical state (phase)

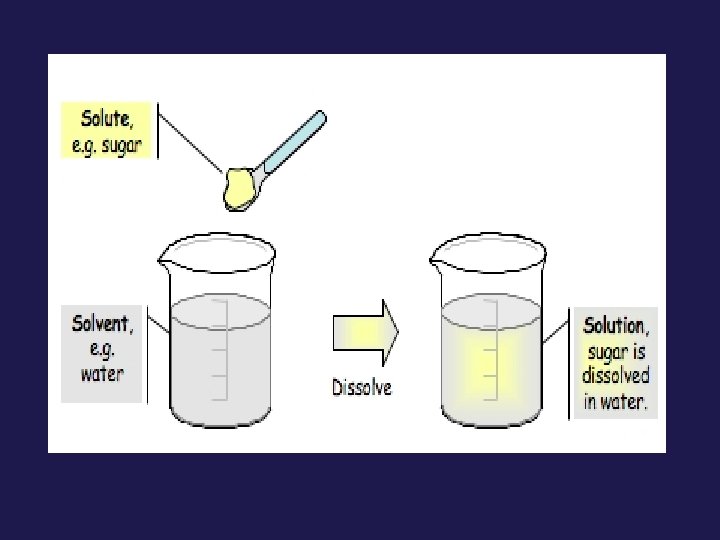
Parts of a Solution • Solute = substance being dissolved • Solvent = dispersing medium that substance is being dissolved in
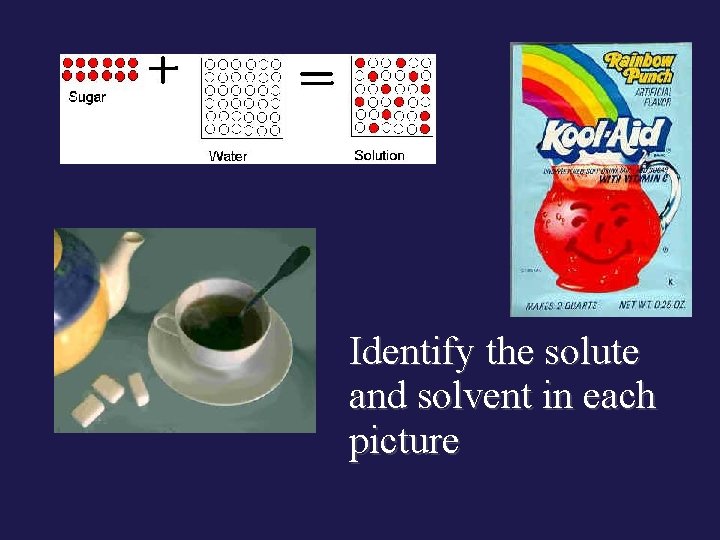
Identify the solute and solvent in each picture
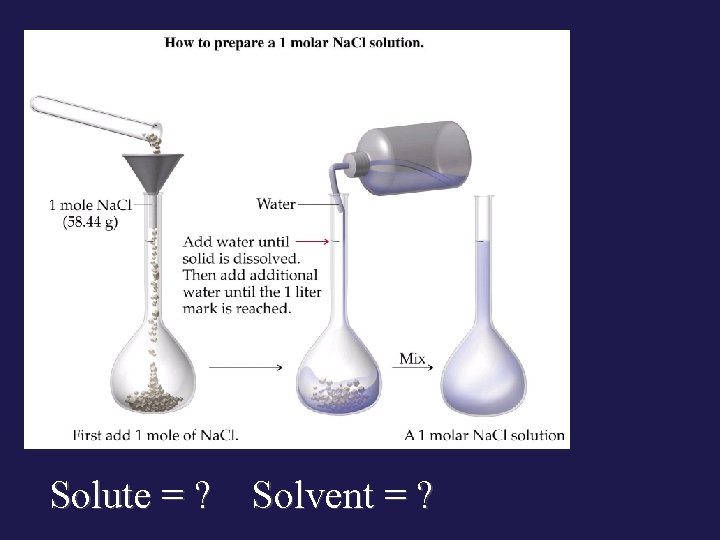
Solute = ? Solvent = ?
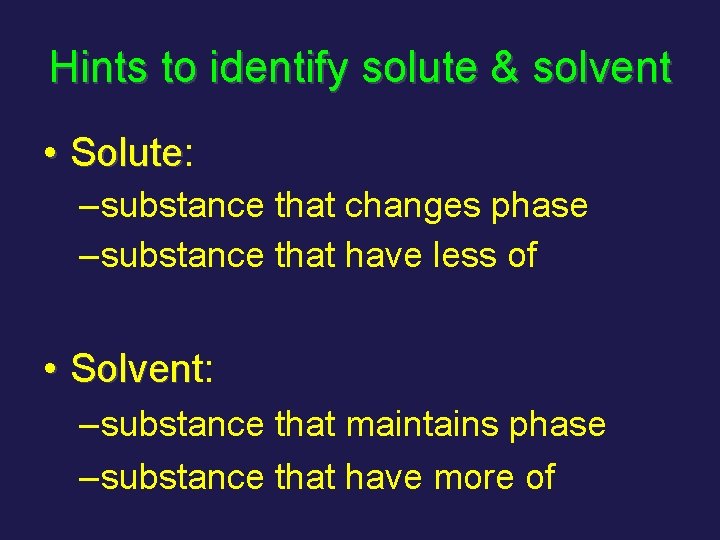
Hints to identify solute & solvent • Solute: Solute – substance that changes phase – substance that have less of • Solvent: Solven – substance that maintains phase – substance that have more of

Aqueous Solutions • Water is solvent • Transition metals form brightly colored solutions
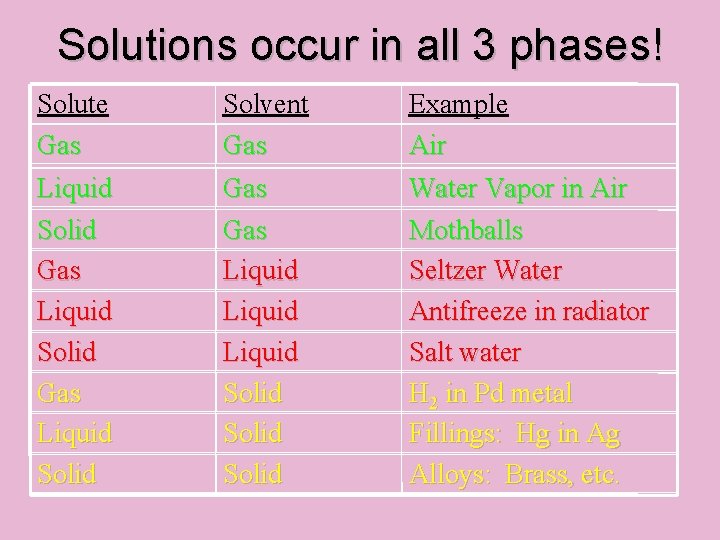
Solutions occur in all 3 phases! Solute Gas Solvent Gas Example Air Liquid Solid Gas Liquid Solid Water Vapor in Air Mothballs Seltzer Water Antifreeze in radiator Salt water H 2 in Pd metal Fillings: Hg in Ag Alloys: Brass, etc.
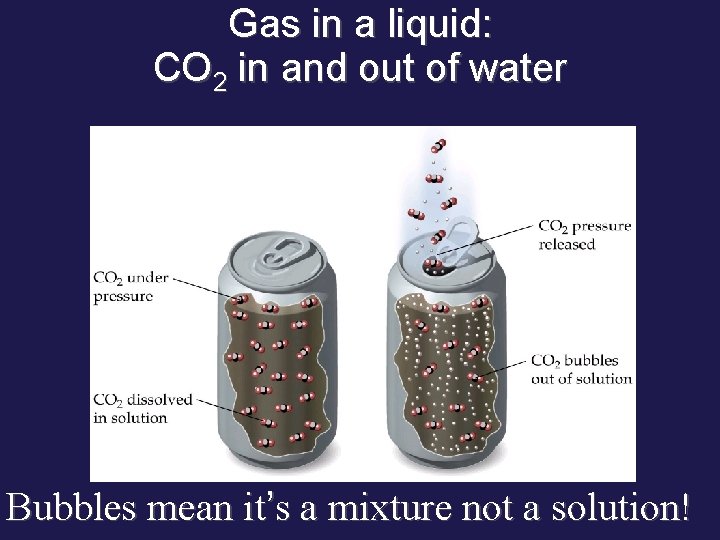
Gas in a liquid: CO 2 in and out of water Bubbles mean it’s a mixture not a solution!

Concentration of Solutions

Concentration • measurement that describes how much solute in given amount of solvent or solution • express concentration using many, many different units – pick the unit according to task at hand

Percent • By mass • By Volume % = Part x Whole 100

Twists • sometimes ask for part or whole, not percent • sometimes give volume/mass of solution • sometimes give volume/mass of solvent and volume/mass of solute Mass solution = Mass solute + Mass solvent

ppm (parts per million) • used when solute is present in very small amounts (Regents board doesn’t follow this rule)
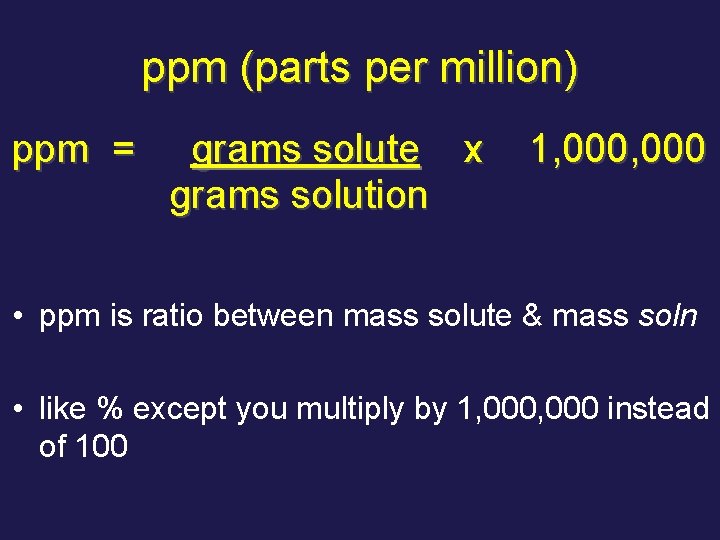
ppm (parts per million) ppm = grams solute x grams solution 1, 000 • ppm is ratio between mass solute & mass soln • like % except you multiply by 1, 000 instead of 100

About 0. 0043 g of O 2 can be dissolved in 100 m. L of water at 20 o. C. Express in ppm. [Memorize: 1 m. L water has mass of 1 g] • ppm = 0. 0043 g 100. 0043 = 43 ppm x 1, 000

CO 2 gas has solubility of 0. 0972 g/100 g water at 40 o. C. Express in parts per million. ppm = 0. 0972 g solute x 100. 0972 g solution = 971 ppm 1, 000

What is the concentration in ppm of a solution with 30. 0 g Na. NO 3 in 70. 0 g water? ppm = 30. 0 g Na. NO 3 100. 0 g solution x 1, 000 = 300, 000 = 3. 0 X 105 ppm

How many grams of KOH are needed to be dissolved in water to make 2000. 0 grams of a 10. 0 ppm solution? 10 ppm = ? grams KOH X 1, 000 2000. 0 g solution ? = 0. 02 grams KOH
- Slides: 23