Solutions Chemistry Unit 10 What Are Solutions Characteristics














- Slides: 28

Solutions Chemistry – Unit 10

What Are Solutions? � Characteristics of Solutions �A substance that dissolves in a solvent is soluble in that solvent. � Sugar is soluble in water �A substance that does not dissolve in a solvent is insoluble in that solvent. � Sand is insoluble in water � Immiscible – two liquids that do not mix � Miscible – two liquids that do mix

� Solvation in Aqueous Solutions � Solvation – process of surrounding solute particles with solvent particles to form a solution � Hydration – solvation in water � “Like dissolves like” is the general rule to determine whether solvation will occur Bonding and polarity of particles and intermolecular forces between particles � Aqueous solutions of ionic compounds � Ex: for sodium chloride, charged ends of water molecules attract positive sodium ions and negative chloride ions � Solvation continues until entire crystal has dissolved and all ions are distributed throughout the solvent � Aqueous � Ex: solutions of molecular compounds sucrose (sugar) dissolves in water b/c it has many O-H bonds, which are polar

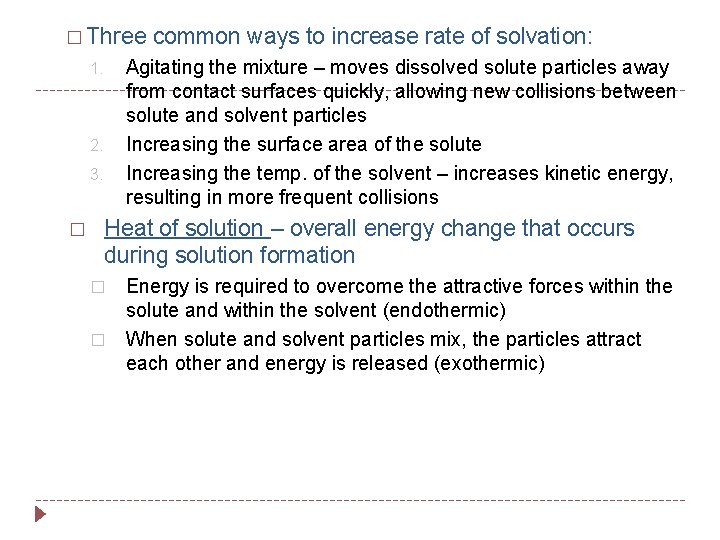
� Three Agitating the mixture – moves dissolved solute particles away from contact surfaces quickly, allowing new collisions between solute and solvent particles Increasing the surface area of the solute Increasing the temp. of the solvent – increases kinetic energy, resulting in more frequent collisions 1. 2. 3. � common ways to increase rate of solvation: Heat of solution – overall energy change that occurs during solution formation � � Energy is required to overcome the attractive forces within the solute and within the solvent (endothermic) When solute and solvent particles mix, the particles attract each other and energy is released (exothermic)

� Solubility – amount of solute that will dissolve in a given amount of solvent at a specified temp. and pressure � Saturated solution - contains the max amount of dissolved solute for a given amount of solvent at a specific temp. and pressure � Unsaturated solution – contains less dissolved solute for a given temp. and pressure (more solute can be dissolved) � Factors That Affect Solubility � Temperature � Many substances are more soluble at high temps than at low temps EXCEPTION – solubility of a gaseous solute decreases as temp increases Example: CO 2 dissolved in soda

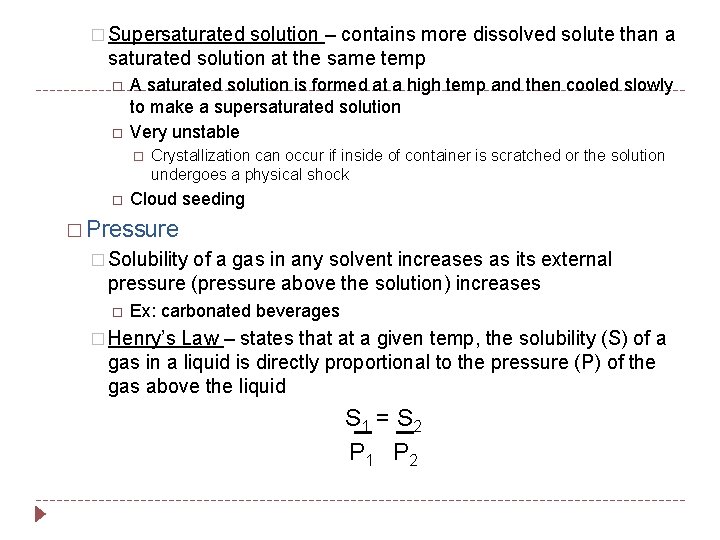
� Supersaturated solution – contains more dissolved solute than a saturated solution at the same temp A saturated solution is formed at a high temp and then cooled slowly to make a supersaturated solution Very unstable Crystallization can occur if inside of container is scratched or the solution undergoes a physical shock Cloud seeding � Pressure � Solubility of a gas in any solvent increases as its external pressure (pressure above the solution) increases Ex: carbonated beverages � Henry’s Law – states that at a given temp, the solubility (S) of a gas in a liquid is directly proportional to the pressure (P) of the gas above the liquid S 1 = S 2 P 1 P 2

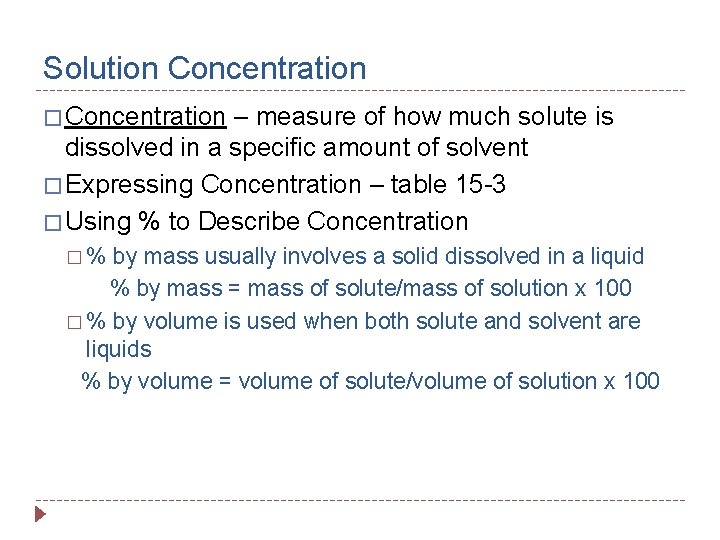
Solution Concentration � Concentration – measure of how much solute is dissolved in a specific amount of solvent � Expressing Concentration – table 15 -3 � Using % to Describe Concentration �% by mass usually involves a solid dissolved in a liquid % by mass = mass of solute/mass of solution x 100 � % by volume is used when both solute and solvent are liquids % by volume = volume of solute/volume of solution x 100

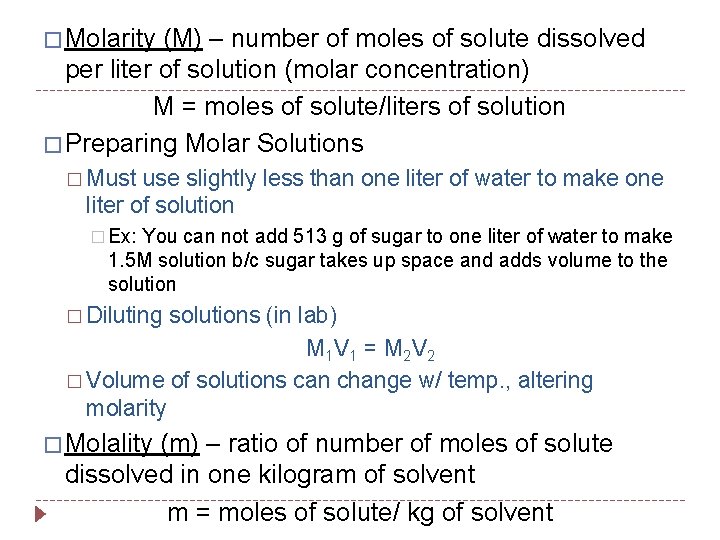
� Molarity (M) – number of moles of solute dissolved per liter of solution (molar concentration) M = moles of solute/liters of solution � Preparing Molar Solutions � Must use slightly less than one liter of water to make one liter of solution � Ex: You can not add 513 g of sugar to one liter of water to make 1. 5 M solution b/c sugar takes up space and adds volume to the solution � Diluting solutions (in lab) M 1 V 1 = M 2 V 2 � Volume of solutions can change w/ temp. , altering molarity � Molality (m) – ratio of number of moles of solute dissolved in one kilogram of solvent m = moles of solute/ kg of solvent

Heterogeneous Mixtures � Suspension – mixture containing particles that settle out if left undisturbed � Gravity acts on suspended particles in a short time � Cornstarch and water is a thixotropic mixture � Settle out cornstarch particles form a solid-like state on the bottom of the container � When stirred the solid-like state quickly begins flowing like a liquid � Colloid – heterogeneous mixture of intermediate size particles (between size of solution particles and suspension particles) � Ex: milk � Can’t separate its components by settling or by filtration

� Brownian motion – jerky, random, and erratic movement of colloid particles � Results from collisions of particles of the dispersion medium w/ dispersed particles � The Tyndall effect – scattering of light caused by dispersed colloid particles � Ex: rays of sunlight passing through smoke; lights through fog

Properties of Acids and Bases � Acidic � Ex: lemon juice � Basic � Ex: solutions taste sour solutions taste bitter and feel slippery soap � Pure water is a nonconductor of electricity, but the addition of an acid or base makes it a conductor � Ions In Solution � Aqueous solutions contain hydrogen ions (H+) and hydroxide ions (OH-) and the relative amounts of the two ions determine whether the solution is acidic, basic, or neutral

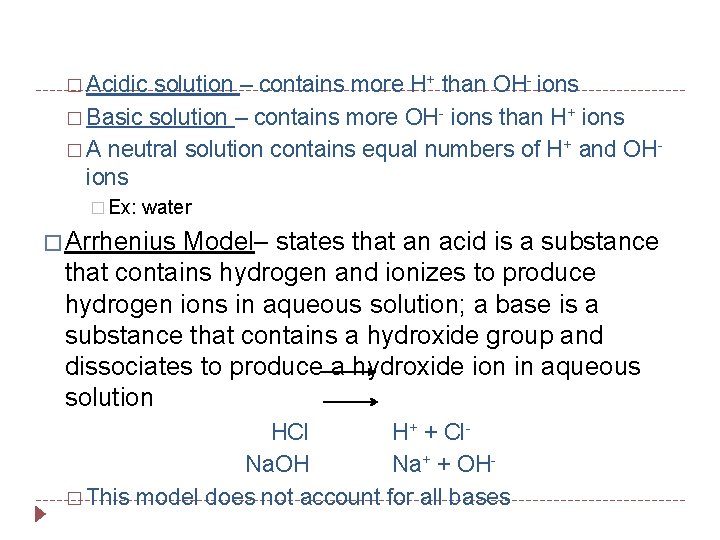
� Acidic solution – contains more H+ than OH- ions � Basic solution – contains more OH- ions than H+ ions � A neutral solution contains equal numbers of H+ and OHions � Ex: water � Arrhenius Model– states that an acid is a substance that contains hydrogen and ionizes to produce hydrogen ions in aqueous solution; a base is a substance that contains a hydroxide group and dissociates to produce a hydroxide ion in aqueous solution HCl H+ + Cl. Na. OH Na+ + OH� This model does not account for all bases

� Bronsted-Lowry Model – an acid is a hydrogen-ion donor and a base is a hydrogen-ion acceptor � When a molecule of acid dissolves in water it donates a H+ ion to a water molecule; the water molecule acts as a base and accepts the H+ ion HX + H 2 O H 3 O + + X � On accepting the H+ ion, the water molecule becomes an acid H 3 O + � X- is a base b/c it has a negative charge and can accept a positive H+ ion � Conjugate acid – species produced when a base accepts a H+ ion from an acid � The base H 2 O accepts a H+ ion from the acid HX and becomes the conjugate acid H 3 O+ � Conjugate base – species that results when an acid donates a H+ ion to a base � The acid HX donates its H+ ion and becomes the conjugate base X-

� Hacky Sack game � Amphoteric – substances that can act as both acids and bases � Ex: water � Substances classified as acids and bases by the Arrhenius model are classified as acids and bases by the Bronsted-Lowry model; however some substances not classified as bases by the Arrhenius model are classified as bases by the Bronsted-Lowry model

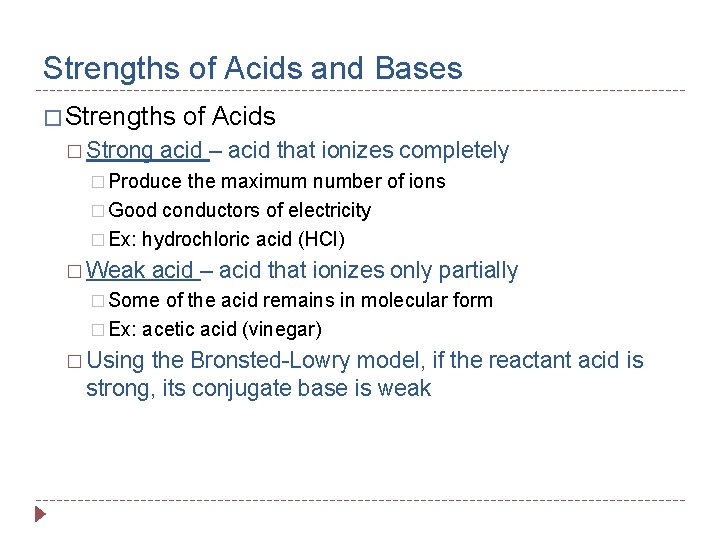
Strengths of Acids and Bases � Strengths � Strong of Acids acid – acid that ionizes completely � Produce the maximum number of ions � Good conductors of electricity � Ex: hydrochloric acid (HCl) � Weak acid – acid that ionizes only partially � Some of the acid remains in molecular form � Ex: acetic acid (vinegar) � Using the Bronsted-Lowry model, if the reactant acid is strong, its conjugate base is weak

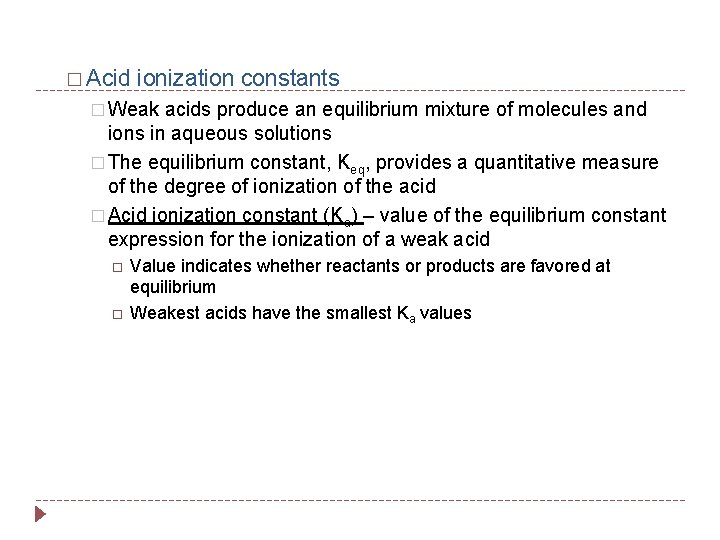
� Acid ionization constants � Weak acids produce an equilibrium mixture of molecules and ions in aqueous solutions � The equilibrium constant, Keq, provides a quantitative measure of the degree of ionization of the acid � Acid ionization constant (Ka) – value of the equilibrium constant expression for the ionization of a weak acid Value indicates whether reactants or products are favored at equilibrium Weakest acids have the smallest Ka values

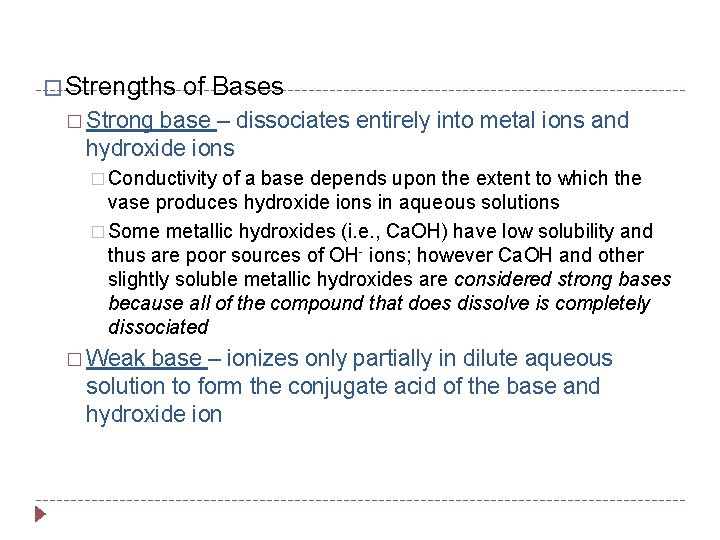
� Strengths of Bases � Strong base – dissociates entirely into metal ions and hydroxide ions � Conductivity of a base depends upon the extent to which the vase produces hydroxide ions in aqueous solutions � Some metallic hydroxides (i. e. , Ca. OH) have low solubility and thus are poor sources of OH- ions; however Ca. OH and other slightly soluble metallic hydroxides are considered strong bases because all of the compound that does dissolve is completely dissociated � Weak base – ionizes only partially in dilute aqueous solution to form the conjugate acid of the base and hydroxide ion

� Base ionization constants � Weak bases also form equilibrium mixtures of molecules and ions in aqueous solutions � Base ionization constant (Kb) – value of the equilibrium constant expression for the ionization of a base � The smaller the value of Kb, the weaker the base � Strong/weak, � For concentrated/dilute? acids and bases, weak/strong and dilute/concentrated have different meanings � Weak or strong refers to the degree to which the acid or base separates into ions

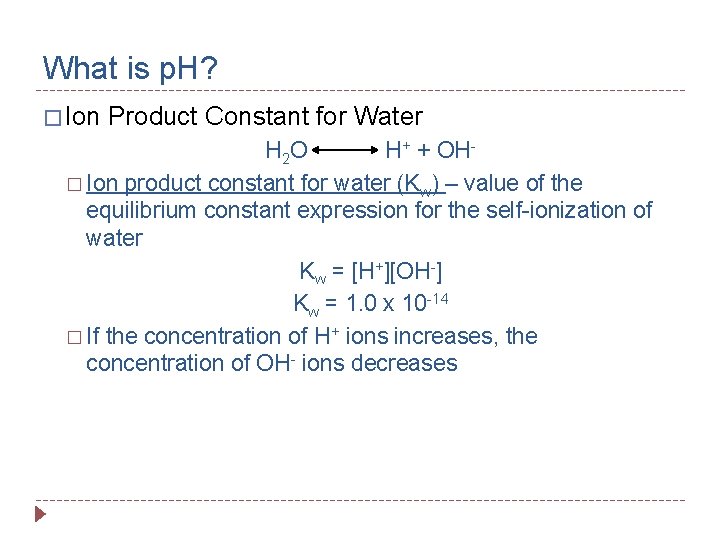
What is p. H? � Ion Product Constant for Water H 2 O H+ + OH� Ion product constant for water (Kw) – value of the equilibrium constant expression for the self-ionization of water Kw = [H+][OH-] Kw = 1. 0 x 10 -14 � If the concentration of H+ ions increases, the concentration of OH- ions decreases

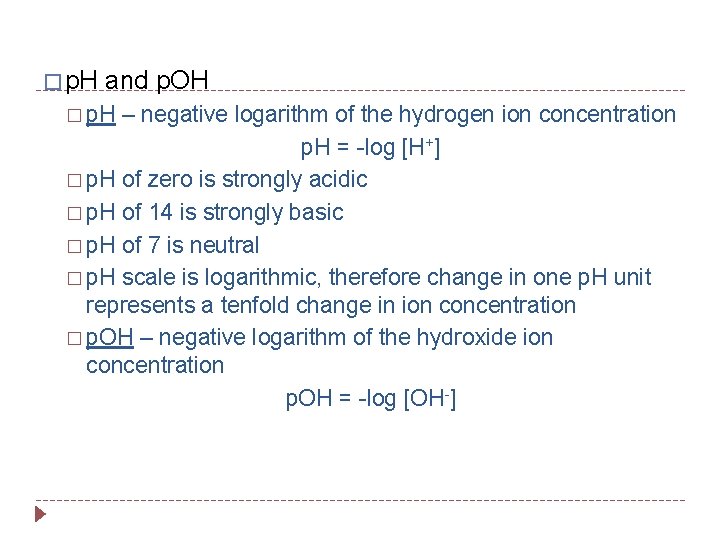
� p. H and p. OH � p. H – negative logarithm of the hydrogen ion concentration p. H = -log [H+] � p. H of zero is strongly acidic � p. H of 14 is strongly basic � p. H of 7 is neutral � p. H scale is logarithmic, therefore change in one p. H unit represents a tenfold change in ion concentration � p. OH – negative logarithm of the hydroxide ion concentration p. OH = -log [OH-]

� p. H + p. OH = 14. 00 � When a strip of p. H paper is dipped into an acidic or basic solution, the color of the paper changes; then the color of the paper is compared to a standard on a chart

Neutralization � Reaction Between Acids and Bases � Neutralization reaction – an acid and a base react in aqueous solution to produce a salt and water � Salt – ionic compound made up of a cation from a base and an anion from an acid � Titration – method for determining concentration of a solution by reacting a known volume of the solution with a solution of known concentration � To find the concentration of an acid solution, titrate the acid solution w/ a solution of a base of known concentration � To find the concentration of a basic solution, titrate a base of unknown concentration w/ an acid of known concentration

� Acid-base indicators – chemical dyes whose colors are affected by acidic and basic solutions � End point – point at which the indicator used in a titration changes color � Some titrations have equivalence points <7 and some have equivalence points >7; it depends on the relative strengths of the reacting acids and bases � Salt Hydrolysis – anions of the dissociated salt accept hydrogen ions from water or the cations of the dissociated salt donate hydrogen ions to water

� Buffered � Control � Blood � Buffers Solutions of p. H is important in your body p. H must be maintained at an average of 7. 4 – solutions that resist changes in p. H when limited amounts of acid or base are added � Buffer is a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid � Buffer solution’s capacity to resist p. H change can be exceeded by the addition of too much acid or base � Buffer capacity – amount of acid or base a buffer solution can absorb w/o a significant change in p. H

Oxidation and Reduction � Electron Transfer and Redox Reactions – reaction in which electrons are transferred from one atom to another � Oxidation – loss of electrons from atoms of a substance � Originally, oxidation referred to a substance combined w/ oxygen � Reduction �LEO – gain of electrons by atoms of a substance the lion says GER – Loss of Electrons is Oxidation; Gain of Electrons GER! is Reduction

� Oxidizing and Reducing Agents � Oxidizing agent – substance that oxidizes another substance by accepting its electrons � The substance that is reduced � Reducing agent – substance that reduces another substance by losing electrons � The substance that is oxidized � Common applications of redox chemistry: � Removal of tarnish from metal � Hydrogen peroxide sanitizes wounds � Chlorine is a strong oxidizer that is used in bleach

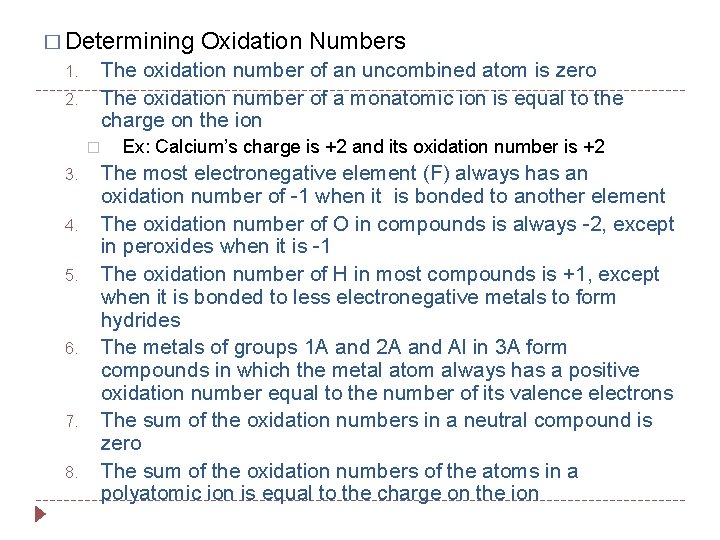
� Determining 1. 2. The oxidation number of an uncombined atom is zero The oxidation number of a monatomic ion is equal to the charge on the ion � 3. 4. 5. 6. 7. 8. Oxidation Numbers Ex: Calcium’s charge is +2 and its oxidation number is +2 The most electronegative element (F) always has an oxidation number of -1 when it is bonded to another element The oxidation number of O in compounds is always -2, except in peroxides when it is -1 The oxidation number of H in most compounds is +1, except when it is bonded to less electronegative metals to form hydrides The metals of groups 1 A and 2 A and Al in 3 A form compounds in which the metal atom always has a positive oxidation number equal to the number of its valence electrons The sum of the oxidation numbers in a neutral compound is zero The sum of the oxidation numbers of the atoms in a polyatomic ion is equal to the charge on the ion

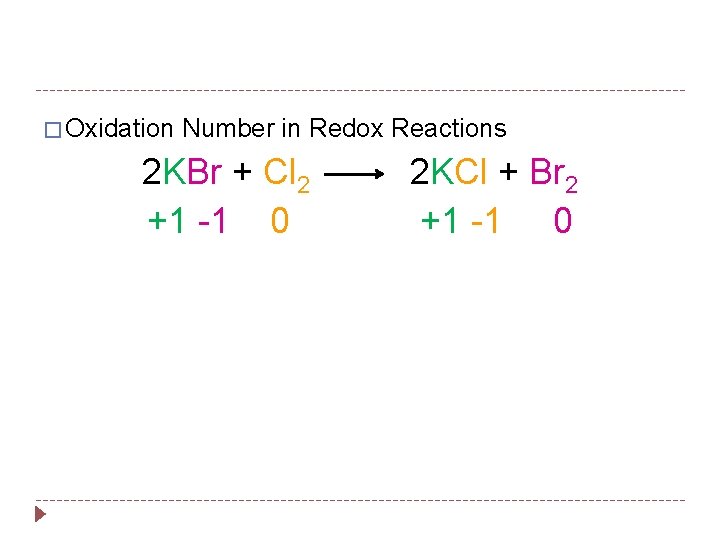
� Oxidation Number in Redox Reactions 2 KBr + Cl 2 +1 -1 0 2 KCl + Br 2 +1 -1 0