Solutions Chapter 15 Pure Impure If a material


























- Slides: 38

Solutions Chapter 15

Pure & Impure Ø If a material is pure, it consists of only a single element or compound Ø If the material is impure, then it is a mixture or contains two or more elements or compounds l l Do not be fooled by labels that claim their product is pure… “pure 100% orange juice” Mixtures are termed either heterogeneous or homogeneous

Mixtures Ø A mixture is a combination of two or more substances in which each substance retains its properties l Most materials we see daily are mixtures • Mixture of elements, compounds or both Ø The components of a mixture can be separated from one another by taking advantage of differences in the components’ physical properties l Solids and liquids can be separated by filter paper (Filtration)

Different Mixtures Ø Remember what the prefixes mean… l l Ø Heterogeneous mixtures are when the different components can be seen as individuals l Ø Hetero: different Homo: same e. g. sand in water, pulp in orange juice, salad dressings Homogeneous mixtures have the same composition throughout, therefore any region of the mixture has the same ratio of substances

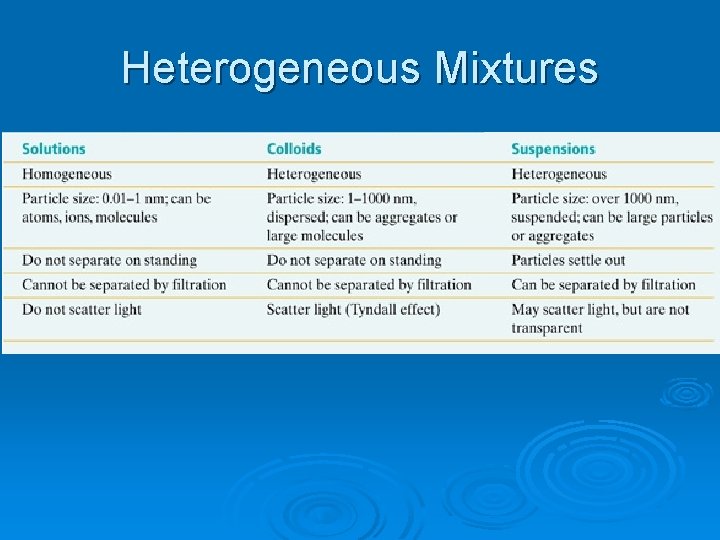
Heterogeneous Mixtures Ø Suspension: a mixture containing particles that settle out if left undisturbed for a period of time l These particles are larger than those found in solvated particles and gravity has greater force on them • ***Particles in a solution are atomic-scale size*** l Fog or jello Ø Colloids: l Mixtures of medium sized particles (between suspensions and solutions l Fine sand, silt in water or tomato juice (milk too)

Heterogeneous Mixtures

Homogeneous Mixtures - Solutions Ø To describe a solution correctly, one must know the lingo or proper terms Ø The component that is present in the largest amount is the solvent l The other component is the solute • Yes you can have more than one solute • Think of it like this… you pour the chocolate syrup (SOLUTE) into the milk (SOLVENT) l In other words, you usually mix the solute into the solvent • Anything that does not mix in the solvent is said to be INSOLUBLE • Have you ever seen something come out of the liquid solution when it cools? This substance is the solute and is known as the PRECIPITATE l Lastly, the term for mixing these components into the solution is known as DISSOLVING!!!

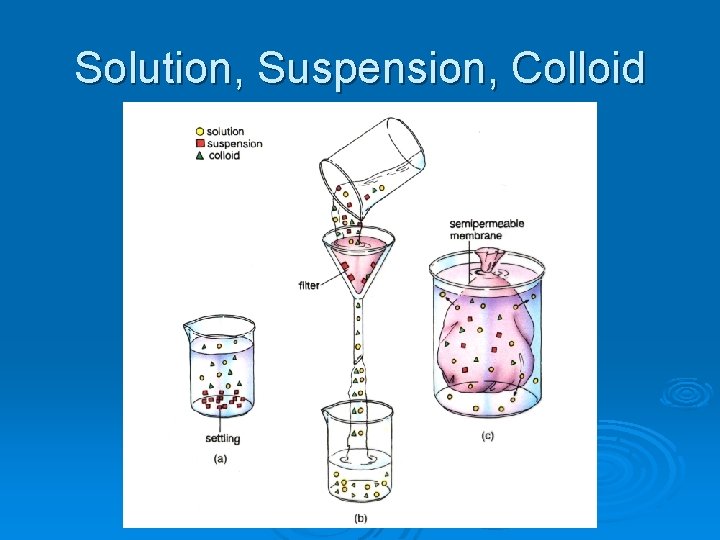
Solution, Suspension, Colloid


Tyndall Effect Put a beam of light through a mixture Ø Reflection of light off undissolved particles Ø Which one is the solution, colloid and suspension? A B C

Solution

Colloid

Suspension

Dissolving Ø How do they dissolve? l The solvent forces the solute apart into individual components and completely surrounds them • This forms the solution and is called solvation Ø Factors that affect the rate of dissolving: l l l Increasing surface area Agitating the solution Heating the solvent

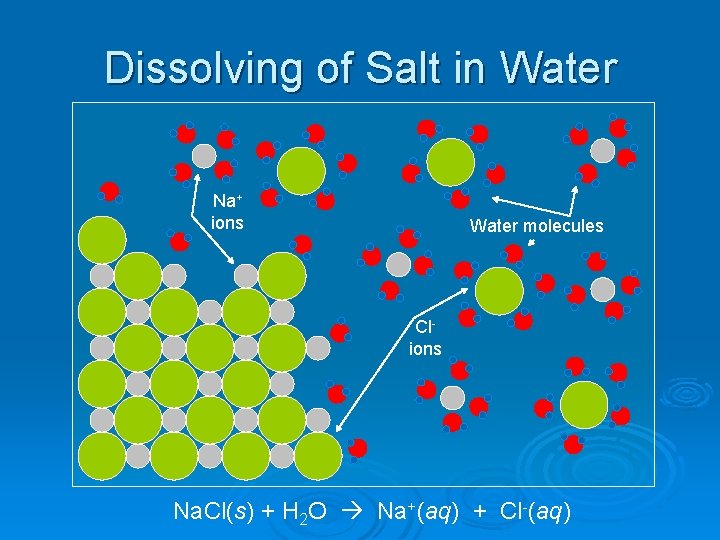
Dissolving of Salt in Water Na+ ions Water molecules Clions Na. Cl(s) + H 2 O Na+(aq) + Cl-(aq)


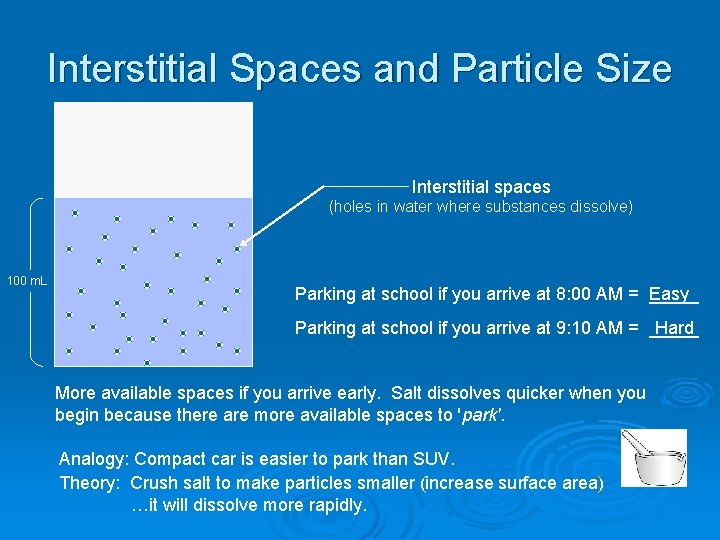
Interstitial Spaces and Particle Size Interstitial spaces (holes in water where substances dissolve) 100 m. L Parking at school if you arrive at 8: 00 AM = _____ Easy Parking at school if you arrive at 9: 10 AM = _____ Hard More available spaces if you arrive early. Salt dissolves quicker when you begin because there are more available spaces to 'park'. Analogy: Compact car is easier to park than SUV. Theory: Crush salt to make particles smaller (increase surface area) …it will dissolve more rapidly. STIR

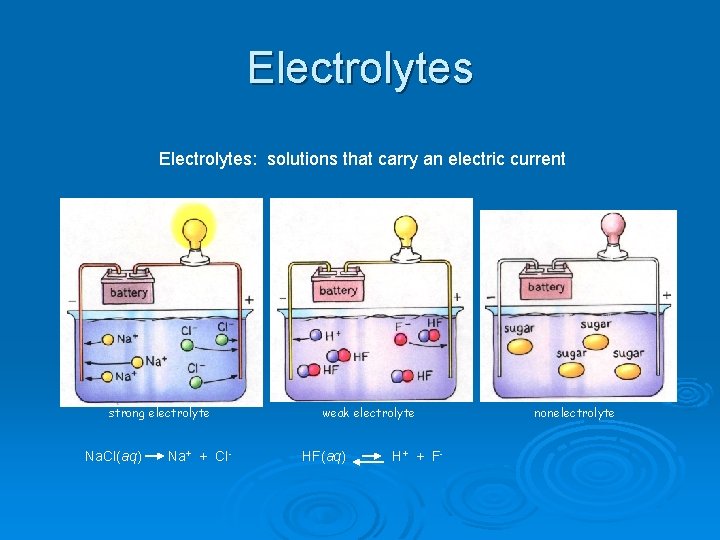
Electrolytes Ø A substance that dissolves in water to give a solution that conducts electric current l These are salts (metal – nonmetal) • Some of the most important chemicals in our body our electrolytes (they are in sport drinks) Ø A nonelectrolyte is a chemical that dissolves in water but does not allow it to conduct electricity

Electrolytes: solutions that carry an electric current strong electrolyte weak electrolyte Na. Cl(aq) Na+ + Cl- HF(aq) H+ + F- nonelectrolyte

General Solubility Guidelines

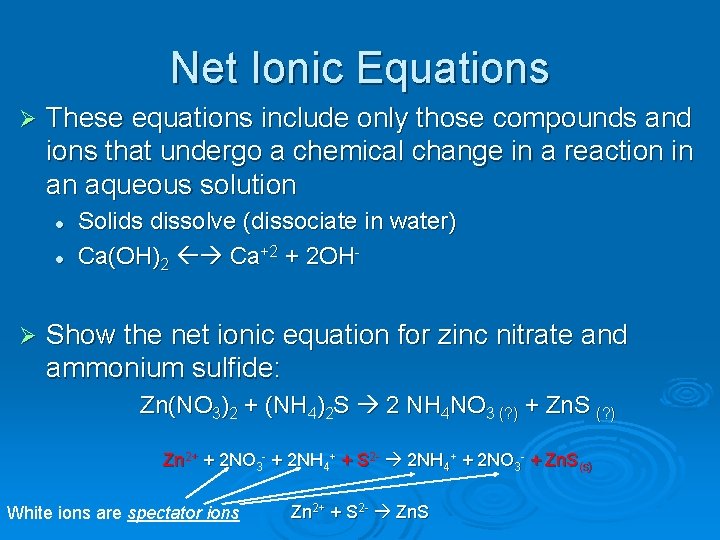
Net Ionic Equations Ø These equations include only those compounds and ions that undergo a chemical change in a reaction in an aqueous solution l l Ø Solids dissolve (dissociate in water) Ca(OH)2 Ca+2 + 2 OH- Show the net ionic equation for zinc nitrate and ammonium sulfide: Zn(NO 3)2 + (NH 4)2 S 2 NH 4 NO 3 (? ) + Zn. S (? ) Zn 2+ + 2 NO 3 - + 2 NH 4+ + S 2 - 2 NH 4+ + 2 NO 3 - + Zn. S(s) White ions are spectator ions Zn 2+ + S 2 - Zn. S

Further Examples Write and Balance the Net Ionic Equations Fe. Cl 3 (aq) + Na. OH (aq) → Fe(OH)3 (s) + Na. Cl (aq) Li. NO 3 + KCl → KNO 3 + Li. Cl

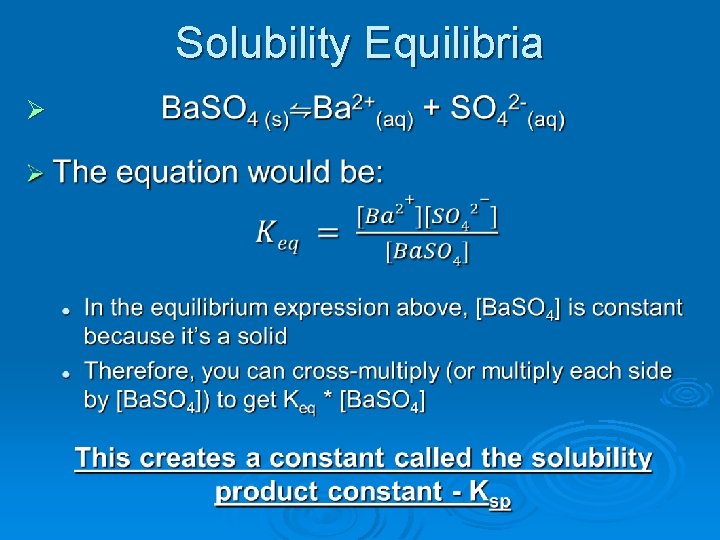
Solubility Equilibria Ø Some ionic compounds will dissociate into their respective ions completely when added to water l Na. Cl(s) → Na+(aq) + Cl-(aq) • These are strong electrolytes Ø Some dissociate then return back to solid form and then dissociate again (and again…) l Ba. SO 4(s) ⇋ Ba 2+(aq) + SO 42 -(aq) • You can solve for the equilibrium constant for this dissociate reactions as well • These are weak electrolytes

Solubility Equilibria Ø

Solubility Product Constant Ø Ksp is very similar to Keq, equilibrium constant for reversible reactions, because it deals with the equilibrium of ions that have not completely disassociated l To solve any problems that would occur asking for solubility of ionic compounds, simply set the Ksp equal to the cation and anion concentrations found in the right side of the equilibrium reaction • If there are coefficients in the balanced reactions, then you will still use them as exponents Ksp = [cation+]C * [anion-]A

Mixing Solutions in Solutions Ø Liquids l l Ø Liquids that don’t want to mix with other liquids like oil and vinegar salad dressing is known as immiscible Therefore, miscible liquid solutions are those that are soluble in one another Gases l Henry’s Law: • The solubility of a gas in a liquid is directly proportional to the partial pressure of that gas on the surface of the liquid l If the gas is escaping from a liquid, it is known as effervescence • CO 2 from sodas, the commercials of alka seltzer

Too Much Stuff Ø

Solution Concentration Ø

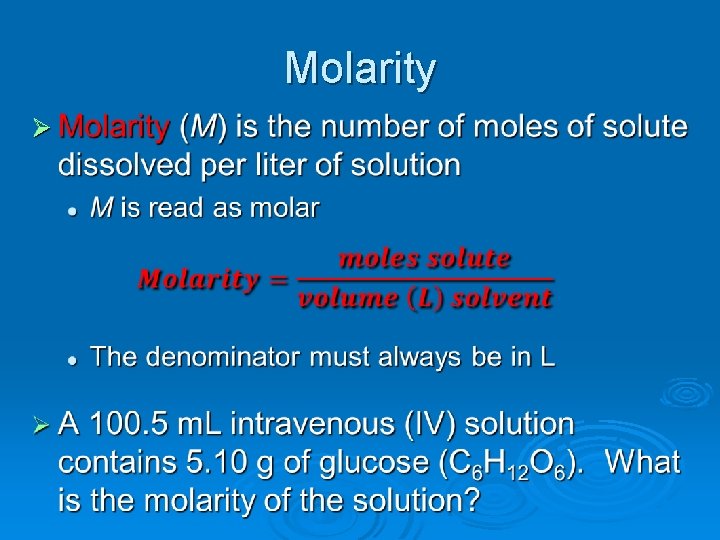
Concentrated vs. Dilute Ø We use these general terms to describe solutions that are saturated or still moderately unsaturated, respectively Ø Here it comes… to get around counting all the individual components, chemists use a unit to measure the number of any particle l MOLE!!!! A mole is equal to 6. 02 x 1023 • Mole is derived from the Latin word for “pile” l The term molarity then refers to the number of moles of solute/liters of solution 602, 000, 000, 000 particles

Molarity Ø

Molarity Practice 1. What is the molarity of a solution containing 21. 0 g Na. Cl in 200 m. L of solution? 2. How do you prepare 250 m. L of a 0. 500 M Na. OH solution?

Preparing Molar Solutions Ø You are now ready to prepare solutions that your teacher used to make for you all morning long l But wait! Do you always use 1 L of solution in those glass reagent bottles? • NO!!!! Ø So here is how you dose it out in smaller quantities…

Preparing Molar Solutions Ø If you need 1. 50 M of glucose but only 100 m. L, how would you prepare this solution. l Ø Normally, you would get 1. 50 moles of glucose (~270 g) and place it in (< 1 L of water) to make 1000 m. L solution. 100 m. L 1. 50 M x 1 L x 270 g C 6 H 12 O 6 = 27. 0 g 1000 m. L 1 L solution

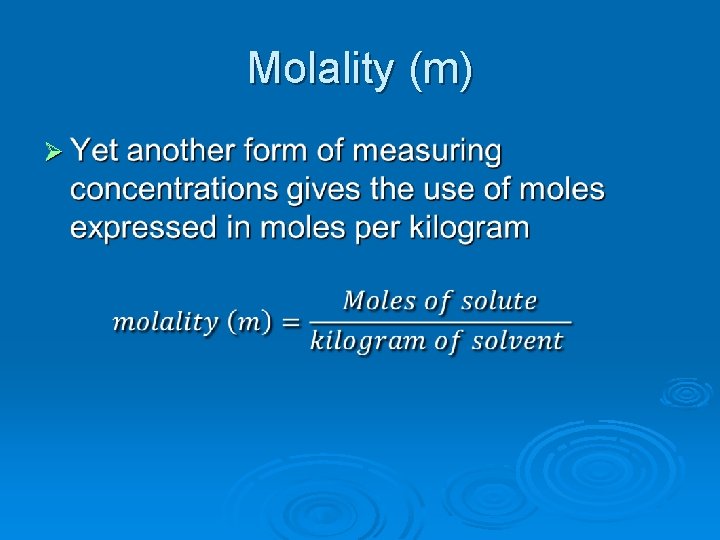
Molality (m) Ø

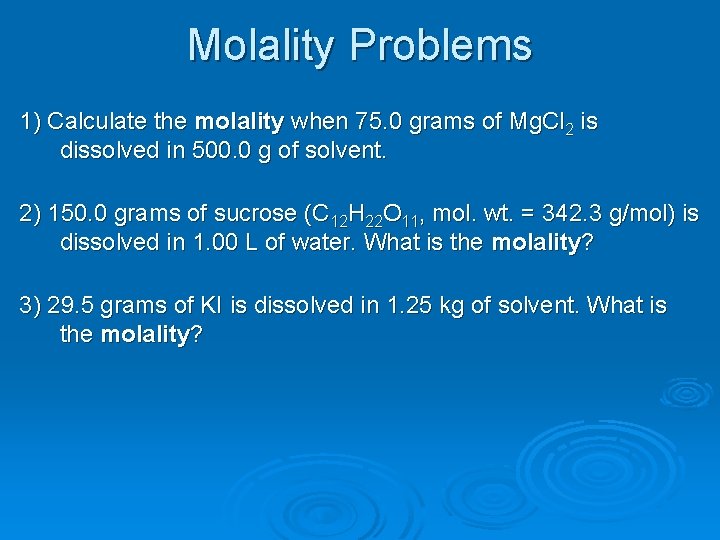
Molality Problems 1) Calculate the molality when 75. 0 grams of Mg. Cl 2 is dissolved in 500. 0 g of solvent. 2) 150. 0 grams of sucrose (C 12 H 22 O 11, mol. wt. = 342. 3 g/mol) is dissolved in 1. 00 L of water. What is the molality? 3) 29. 5 grams of KI is dissolved in 1. 25 kg of solvent. What is the molality?

Preparing Solutions Ø Diluting Solutions l When making solutions from concentrated stock supplies, you can dilute it down to make it a lower concentration Ø Because the total number of moles of solute does not change, only the volume of the solution M 1 V 1 = M 2 V 2 Where M 1 & V 1 represent the molarity and volume of the stock solution and M 2 & V 2 represent the molarity and volume of the dilute solution

Dilution Problems 1. Your teacher needs to make a 0. 500 M solution of HCl from concentrated 12. 0 M HCl. If the volume of the dilute needs to be 500 m. L, then how many m. L of the concentrated does he need to mix with how much water? (Remember the final total volume is 500 m. L). 2. It is necessary to make a 0. 500 M solution of HCl from 500. 0 m. L of a 3. 00 M solution of HCl. What is the volume of the new solution? 3. What is the molarity of a solution which has a volume of 1500. 0 m. L if it was obtained by diluting 250. 0 m. L of a 6. 0 M solution of H 2 SO 4? 4. Your teacher needs to make 500. 0 m. L of a 3. 00 M solution of H 2 SO 4. Concentrated H 2 SO 4 from the chemical company is 18. 0 M. How many m. L of the concentrated acid is needed to dilute with how much water to make this solution?

Colligative Properties Ø The colligative properties of a solution depend primarily on the number of solute particles present rather than the kind of particles. l There are four colligative properties: • • 1. Boiling-point elevation 2. Freezing-Point depression 3. Vapor pressure 4. Osmotic pressure Water boils at 100°C but what happens when you add salt? What if you boil water at very high altitudes?