Solutions ch 16 n Solution a homogeneous mixture









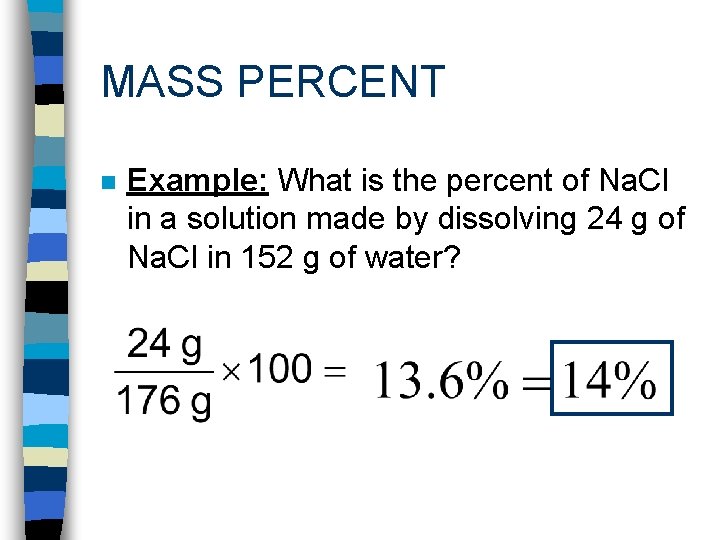





- Slides: 34


Solutions (ch. 16) n Solution – a homogeneous mixture of pure substances n The SOLVENT is the medium in which the SOLUTES are dissolved. (The solvent is usually the most abundant substance. ) – Example: • Solution: Salt Water • Solute: Salt • Solvent: Water

The process of dissolution is favored by: 1) A decrease in the energy of the system (exothermic) 2) An increase in the disorder of the system (entropy)

Liquids Dissolving in Liquids that are soluble in one another (“mix”) are MISCIBLE. – “LIKE dissolves LIKE” n n POLAR liquids are generally soluble in other POLAR liquids. NONPOLAR liquids are generally soluble in other NONPOLAR liquids. LIKE DISSOLVES LIKE : demo

Factors affecting rate of dissolution: think iced tea vs. hot tea & the type of sugar you use: cubes or granulated 1) Surface area / particle size – Greater surface area, faster it dissolves 2) Temperature – Most solids dissolve faster @ higher temps 3) Agitation – Stirring/shaking will speed up dissolution

Saturation: a solid solute dissolves in a solvent until the soln is SATURATED n n n Unsaturated solution – is able to dissolve more solute Saturated solution – has dissolved the maximum amount of solute Supersaturated solution – has dissolved excess solute (at a higher temperature). Solid crystals generally form when this solution is cooled.

ROCK CANDY, YUM!! n

Applying Concepts QUESTION n When the crystallization has stopped, will the solution be saturated or unsaturated? n answer

ANSWER: SATURATED n Solution has the maximum amount of solute for a given quantity of solvent at a constant temperature and pressure.

SOLUBILITY n Solubility = the amount of solute that will dissolve in a given amount of solvent

Factors Affecting Solubility n n n The nature of the solute and solvent: different substances have different solubilities Temperature: many solids substances become more soluble as the temp of a solvent increases; however, gases are less soluble in liquids at higher temps. Pressure: Only affects the solubility of gases. As pressure increases, the solubility of gases increases.

Concentration of Solution n Concentration refers to the amount of solute dissolved in a solution.

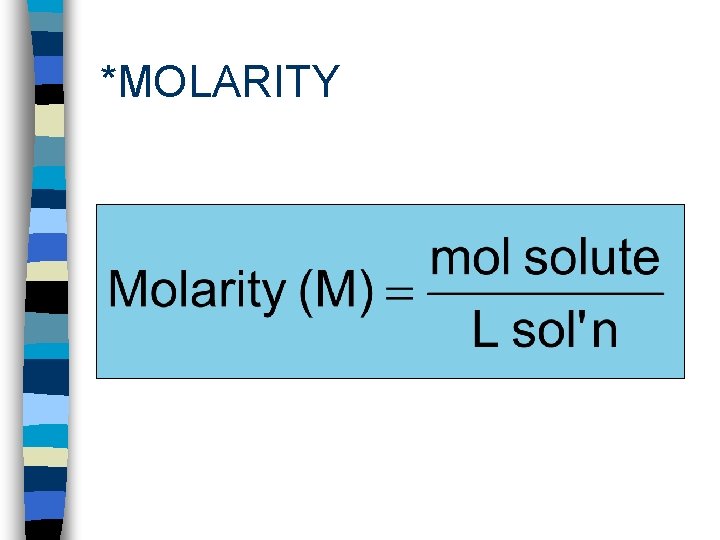
*MOLARITY

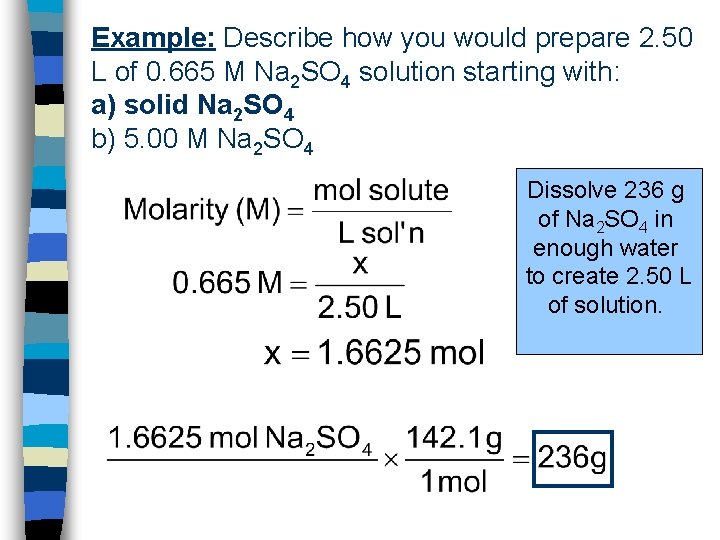
Example: Describe how you would prepare 2. 50 L of 0. 665 M Na 2 SO 4 solution starting with: a) solid Na 2 SO 4 b) 5. 00 M Na 2 SO 4 Dissolve 236 g of Na 2 SO 4 in enough water to create 2. 50 L of solution.

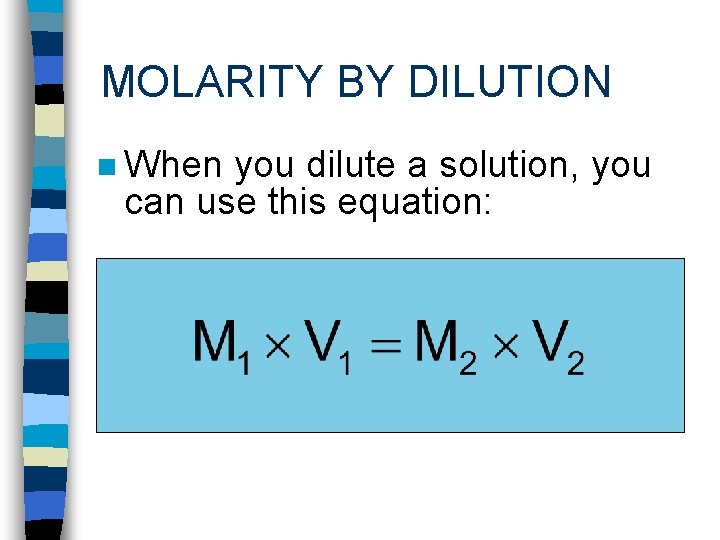
MOLARITY BY DILUTION n When you dilute a solution, you can use this equation:

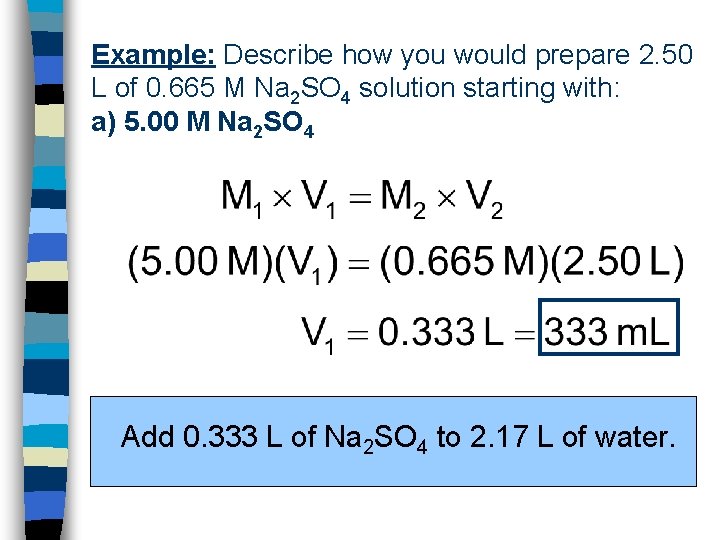
Example: Describe how you would prepare 2. 50 L of 0. 665 M Na 2 SO 4 solution starting with: a) 5. 00 M Na 2 SO 4 Add 0. 333 L of Na 2 SO 4 to 2. 17 L of water.

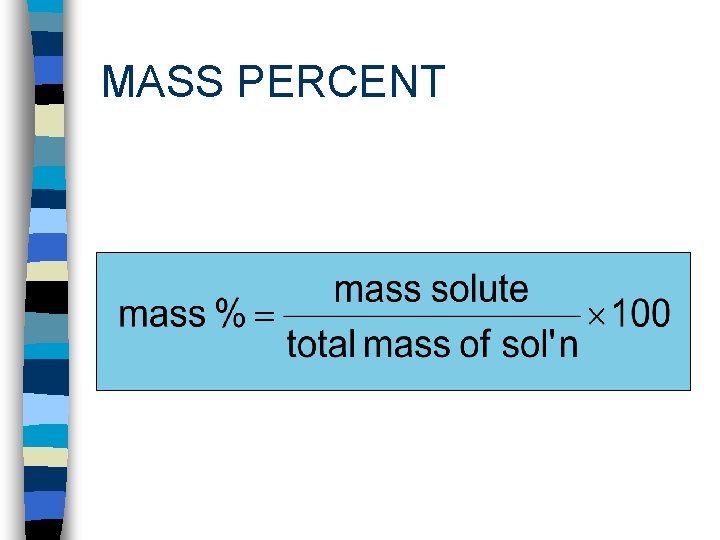
MASS PERCENT
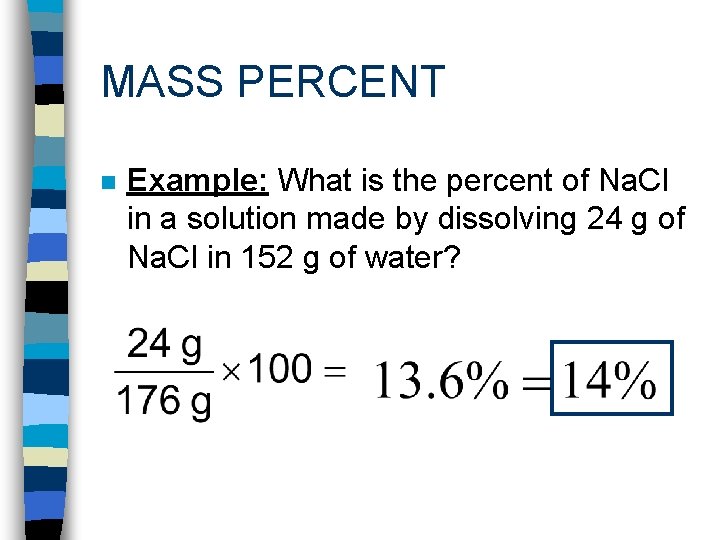
MASS PERCENT n Example: What is the percent of Na. Cl in a solution made by dissolving 24 g of Na. Cl in 152 g of water?

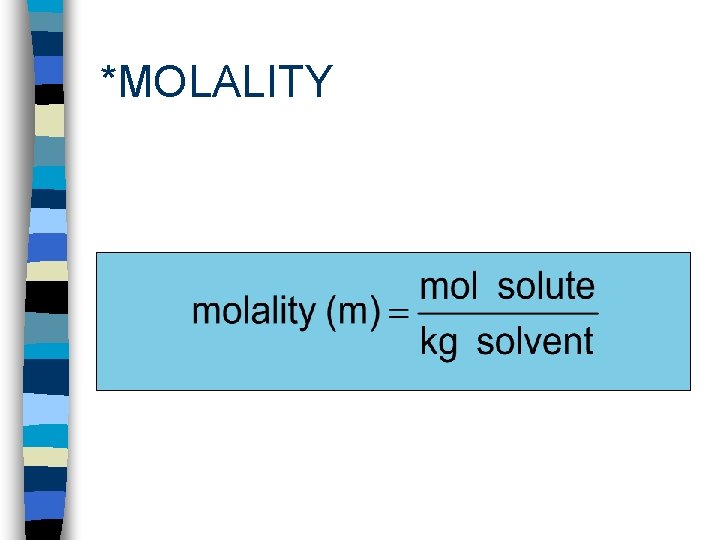
*MOLALITY

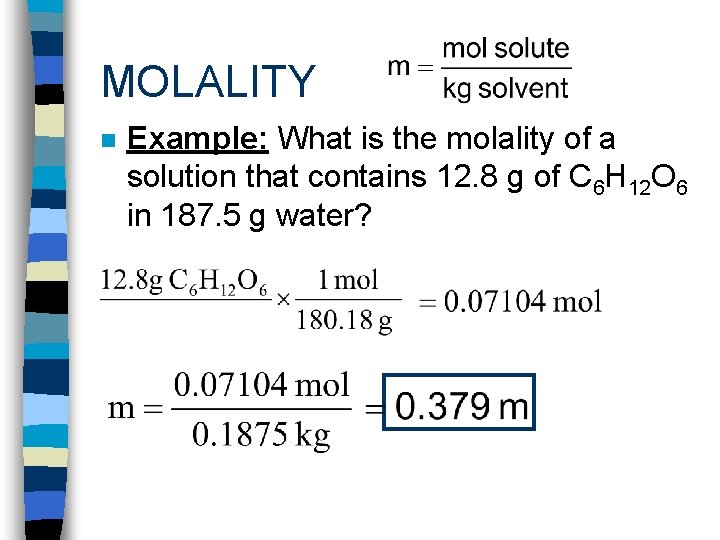
MOLALITY n Example: What is the molality of a solution that contains 12. 8 g of C 6 H 12 O 6 in 187. 5 g water?

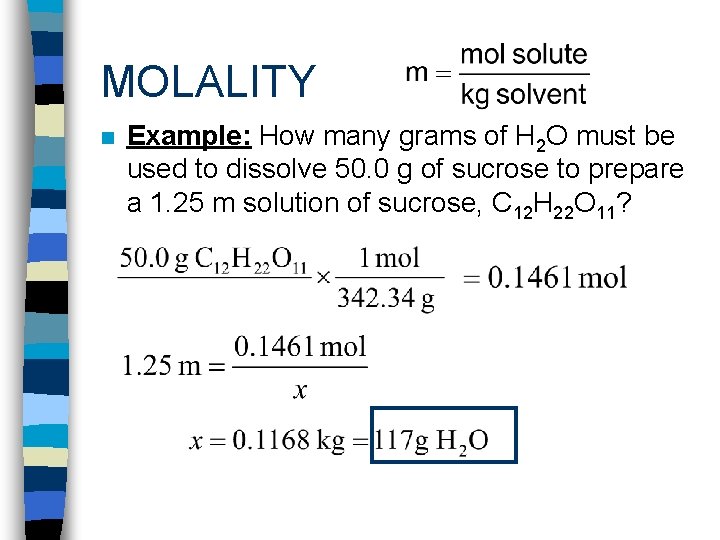
MOLALITY n Example: How many grams of H 2 O must be used to dissolve 50. 0 g of sucrose to prepare a 1. 25 m solution of sucrose, C 12 H 22 O 11?

Colligative Properties of Solutions (chapter 16) Colligative properties = physical properties of solutions that depend on the # of particles dissolved, not the kind of particle.

Colligative Properties n n Lowering vapor pressure Raising boiling point Lowering freezing point Generating an osmotic pressure

2 to focus on… n n Lowering vapor pressure Raising boiling point Lowering freezing point Generating an osmotic pressure

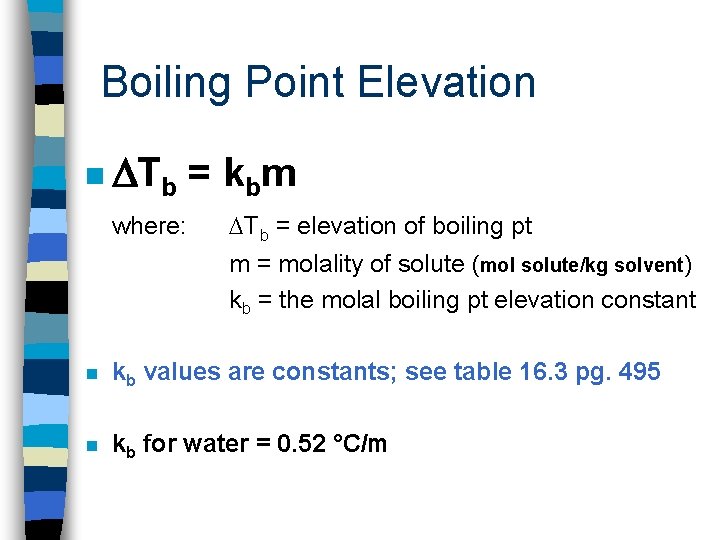
Boiling Point Elevation n a solution that contains a nonvolatile solute has a higher boiling pt than the pure solvent; the boiling pt elevation is proportional to the # of moles of solute dissolved in a given mass of solvent. Like when adding salt to a pot of boiling water to make pasta

Boiling Point Elevation n Tb where: = k bm Tb = elevation of boiling pt m = molality of solute (mol solute/kg solvent) kb = the molal boiling pt elevation constant n kb values are constants; see table 16. 3 pg. 495 n kb for water = 0. 52 °C/m

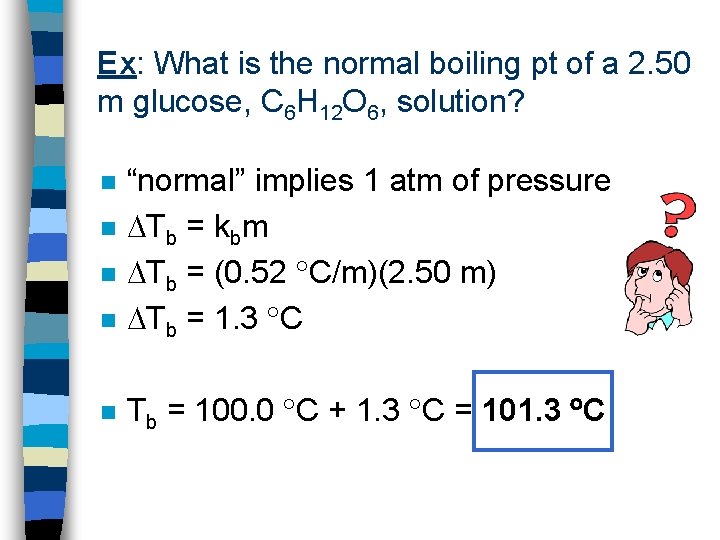
Ex: What is the normal boiling pt of a 2. 50 m glucose, C 6 H 12 O 6, solution? n “normal” implies 1 atm of pressure Tb = kbm Tb = (0. 52 C/m)(2. 50 m) Tb = 1. 3 C n Tb = 100. 0 C + 1. 3 C = 101. 3 C n n n

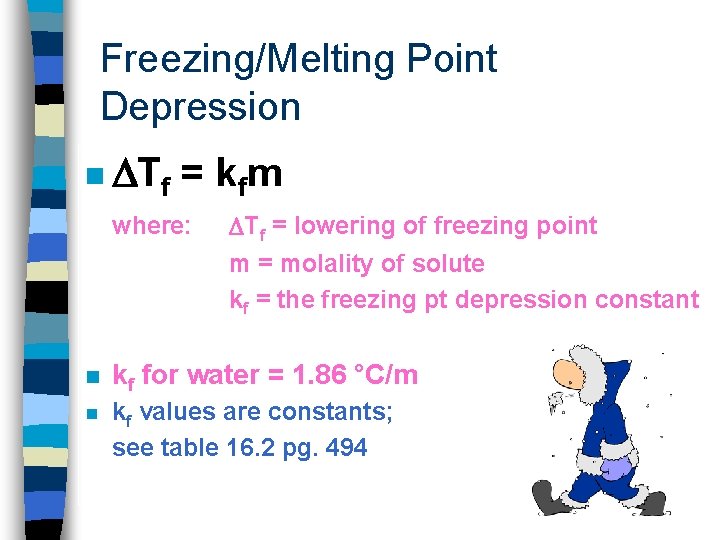
Freezing/Melting Point Depression n The freezing point of a solution is always lower than that of the pure solvent. Like when salting roads in snowy places so the roads don’t ice over or when making ice cream

Freezing/Melting Point Depression n Tf = k fm where: Tf = lowering of freezing point m = molality of solute kf = the freezing pt depression constant n kf for water = 1. 86 °C/m n kf values are constants; see table 16. 2 pg. 494

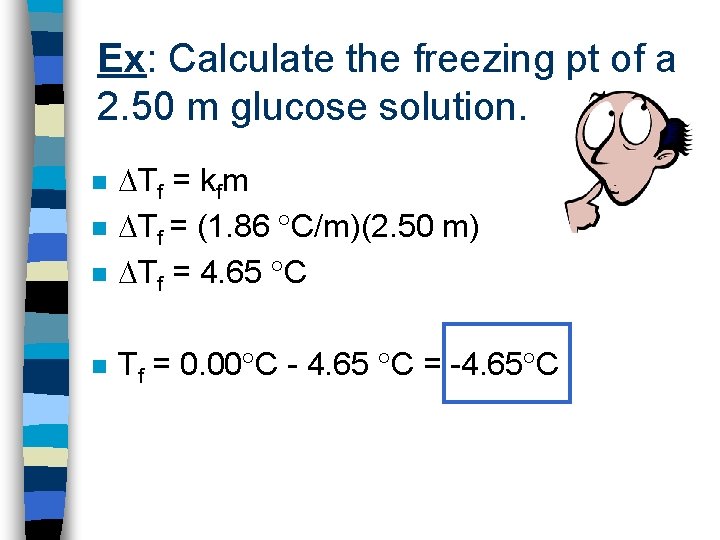
Ex: Calculate the freezing pt of a 2. 50 m glucose solution. n Tf = kfm Tf = (1. 86 C/m)(2. 50 m) Tf = 4. 65 C n Tf = 0. 00 C - 4. 65 C = -4. 65 C n n

Electrolytes and Colligative Properties • Colligative properties depend on the # of particles present in solution. • Because ionic solutes dissociate into ions, they have a greater effect on freezing pt and boiling pt than molecular solids of the same molal conc.

Electrolytes and Colligative Properties n For example, the freezing pt of water is lowered by 1. 86°C with the addition of any molecular solute at a concentration of 1 m. – Such as C 6 H 12 O 6, or any other covalent compound n However, a 1 m Na. Cl solution contains 2 molal conc. of IONS. Thus, the freezing pt depression for Na. Cl is 3. 72°C…double that of a molecular solute. – Na. Cl Na+ + Cl- (2 particles)

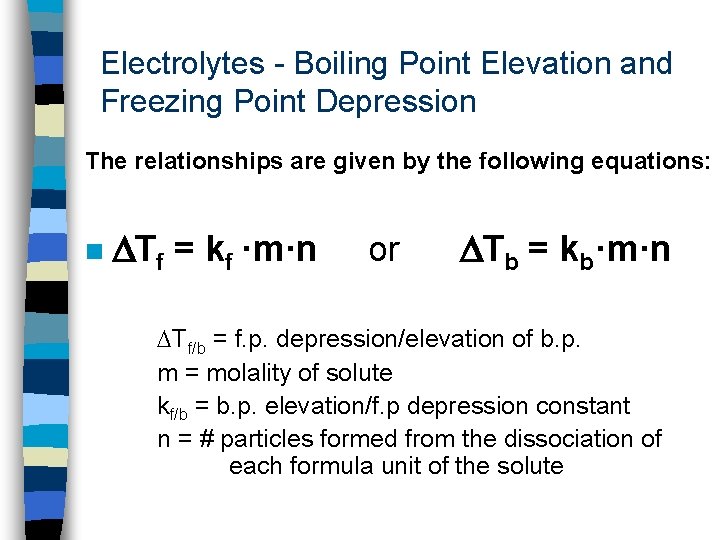
Electrolytes - Boiling Point Elevation and Freezing Point Depression The relationships are given by the following equations: n Tf = kf ·m·n or Tb = kb·m·n Tf/b = f. p. depression/elevation of b. p. m = molality of solute kf/b = b. p. elevation/f. p depression constant n = # particles formed from the dissociation of each formula unit of the solute

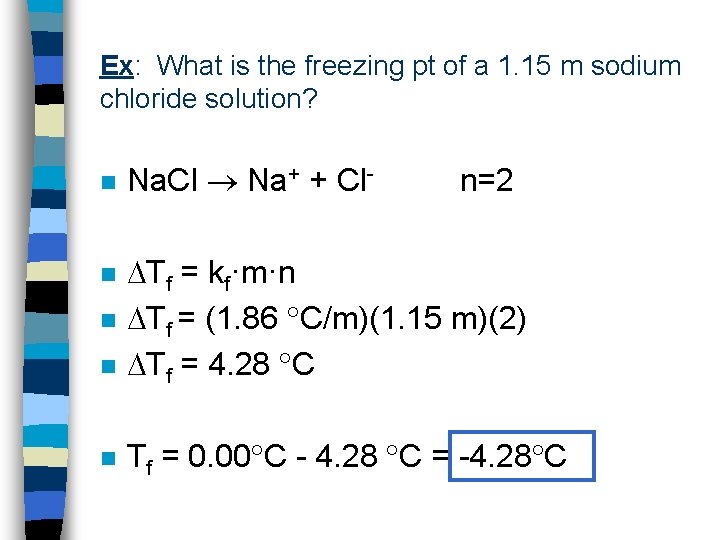
Ex: What is the freezing pt of a 1. 15 m sodium chloride solution? n Na. Cl Na+ + Cl- n=2 n Tf = kf·m·n Tf = (1. 86 C/m)(1. 15 m)(2) Tf = 4. 28 C n Tf = 0. 00 C - 4. 28 C = -4. 28 C n n