Solutions are Homogeneous Usually clear liquids Can vary








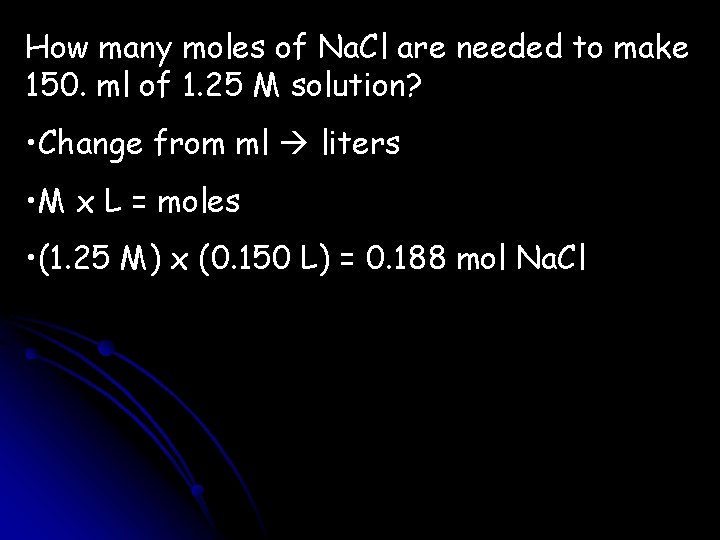












- Slides: 24

Solutions are: • Homogeneous • Usually clear (liquids) • Can vary in concentration • Can not separate by filtration • Will not separate by standing • Made up of at least two different substances • Can separate using a physical change

Solutions – samples containing dissolved substances that can be solid, liquid or gas. Solvent – the dissolving medium. “The thing that the stuff dissolves in. ” Solute – the dissolving particles. “The thing that dissolves. ” Aqueous solution – a solution that has water as the solvent. An aqueous salt solution – water is the solvent, salt is the solute

Factors affecting solvation (how fast things dissolve): • Increasing the heat or temperature • Stirring or agitation • Increasing the surface area of the particles or making the particles smaller

One way to describe compounds: • Polar (ionic) – made up of ions; made up of metals and nonmetals; positive and negative ions • Nonpolar (covalent) – made up of nonmetals, no charged particles Likes Dissolve Likes • Polar solutes dissolve in polar solvents • Nonpolar solutes dissolve in Nonpolar solvents

Miscible – two liquids are miscible if they will dissolve in each other Immiscible – two liquids are immiscible if two liquids will not dissolve in each other. Polar/polar and non polar/nonpolar solutions are miscible. Polar/nonpolar solutions are immiscible. Oil/water – immiscible Water/ food coloring - miscible

Solubility – the amount of substance that can dissolve in a given amount of solvent at a given temperature. Saturated Solution – contains the maximum amount of solute for a given temperature and a given amount of solvent. Unsaturated Solution – contains less than the maximum amount of solute. Supersaturated Solution – contains more solute than it can theoretically hold at a given temperature. It is an unstable solution, and will very easily turn into a solid.

In general, the solubility of solids in liquids increases as the temperature of the solution increases. The solubility of gases in liquids decreases as the temperature of the solution increases. That explains the shape of the solubility graph – most lines curve up. The lines that curve down are gases.

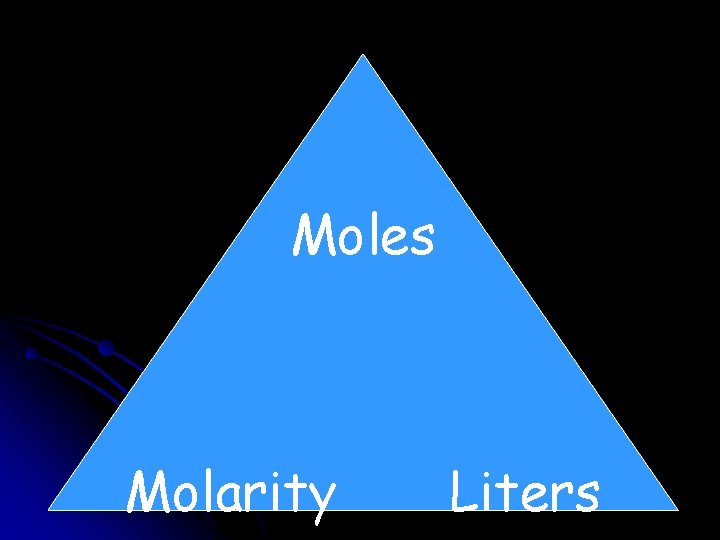
Concentration of a Solution How much solute is dissolved in a given quantity of solvent. The most common unit of concentration in chemistry is MOLARITY. Molarity = (mole of solute/liters of solution) M = mol/liters A 4. 00 M solution contains 4. 00 moles of solute for every liter of solution (2. 00 moles for every 0. 500 liters, 8. 00 moles for every 2. 00 liters, …)
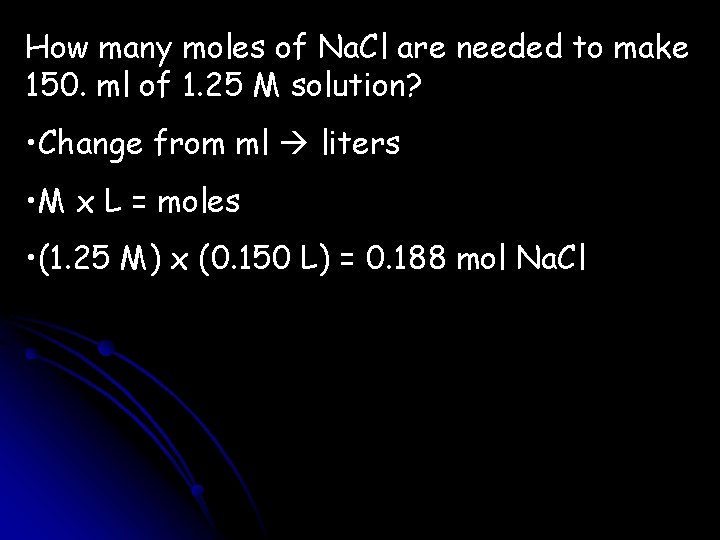
How many moles of Na. Cl are needed to make 150. ml of 1. 25 M solution? • Change from ml liters • M x L = moles • (1. 25 M) x (0. 150 L) = 0. 188 mol Na. Cl

Moles Molarity Liters

1. What volume of solution is needed to turn 17. 3 grams of Na. NO 3 into a 1. 35 M Na. NO 3 solution? 2. How many grams of Na. Cl are needed to make 145 ml of 0. 750 M Na. Cl? 3. What is the molarity of 243 grams of Li. NO 3 dissolved to make 1458 ml of solution? 4. What is the molarity of a solution when 13. 5 grams of Na. Cl is dissolved to make 125 ml of solution?

1. 17. 3 g Na. NO 3 1 mol Na. NO 3 = 0. 2035… mol Na. NO 3 85. 0 g Na. NO 3 (0. 2035… mol Na. NO 3/1. 35 M Na. NO 3) = 0. 151 l solution 2. 0. 145 l soln x 0. 750 M Na. Cl = 0. 10875 mol Na. Cl 58. 5 g Na. Cl = 6. 36 g Na. Cl 1 mol Na. Cl 3. 243 g Li. NO 3 1 mol Li. NO 3 = 3. 526… mol Li. NO 3 68. 9 g Li. NO 3 (3. 526… mol Li. NO 3/1. 458 l soln) = 2. 42 M Li. NO 3 4. 13. 5 g Na. Cl 1 mol Na. Cl = 0. 23076… mol Na. Cl 58. 5 g Na. Cl (0. 23076… mol Na. Cl/0. 125 l soln) = 1. 85 M Na. Cl

Another unit of concentration that is used in the healthcare fields is: % concentration (w/v) or (v/v) w stands for weight, v stands for volume. (amt. of solute/amt. of solution) x 100% What is the % concentration of 18. 5 grams of Na. Cl dissolved to make 125 ml of solution? (18. 5 g Na. Cl/125 ml solution) x 100 % = 14. 8 % (w/v)

1. What is the % concentration (v/v) of a solution made by dissolving 12. 3 ml of oil in 85. 0 ml of gasoline? 2. What mass of Na. OH is needed to dissolve in 275 ml of solution to have a 8. 03 % (w/v) solution? 3. What volume of solution is needed to 4. 56 g of Na. Cl into a 15. 8 % (w/v) solution?

1. 12. 3 ml oil x 100 % = 14. 5 % (v/v) 85. 0 ml gas 2. X grams Na. OH x 100 % = 8. 04 % 275 ml soln X g Na. OH = 0. 0804 X g Na. OH = (0. 0804 x 275) 275 ml soln X = 22. 1 g Na. OH 3. 4. 56 g Na. Cl x 100 % = 15. 8 %(w/v) X ml soln 4. 56 g Na. Cl = 0. 158 4. 56 g = 0. 158 x X ml soln X = 4. 56/ 0. 158 = 28. 9 ml soln

When diluting a solution, the amount of solute remains constant, only the volume of the solution is changed. A stock solution is an essentially unlimited amount of a solution at a given concentration. M 1 V 1 = M 2 V 2 M 1 = concentration of solution (initial) M 2 = concentration of solution (final) V 1 = volume of solution (initial) V 2 = volume of solution (final)

It is very important when working dilution problems to make sure all of the information is properly labeled: get the right concentration paired with the right volume. There are three different volumes in a dilution problem: V 1 = starting volume V 2 = ending volume V 2 -V 1 = amount of solvent added in the dilution.

1. What is the starting concentration of Na. Cl if 14. 6 ml of solution is diluted to make 263. 2 ml of 0. 137 M Na. Cl? 2. What is the final concentration of the solution if 17. 5 ml of 2. 61 M Na. OH is diluted to make 250. 0 ml of solution? 3. How do you make 125 ml of 0. 135 M HCl from 3. 62 M HCl?

1. M 1 V 1 = M 2 V 2 M 1(14. 6) = (0. 137)(263. 2) M 1 = (0. 137 x 263. 2)/14. 6 = 2. 47 M Na. Cl 2. M 1 V 1 = M 2 V 2 (17. 5)(2. 61) = (250. 0)M 2 = (17. 5 x 2. 61)/250. 0 = 0. 183 M Na. OH 3. M 1 V 1 = M 2 V 2 (0. 135)(125) = (3. 62)V V 2 = (0. 135 x 125)/3. 62 = 4. 66 ml of 3. 62 M HCl 125 ml – 4. 66 ml = 120. ml of water

Colligative Properties of a solution that depend on the number of particles dissolved in the solvent. 1. Boiling Point (BP) 2. Freezing Point (FP) 3. Vapor Pressure (VP) 4. Osmosis

As the number of particles in the solution increases: 1. The BP increases - important 2. The FP decreases - important 3. The VP decreases 4. The solvent always flows from the solution with the smaller concentration towards the solution with the higher concentration.

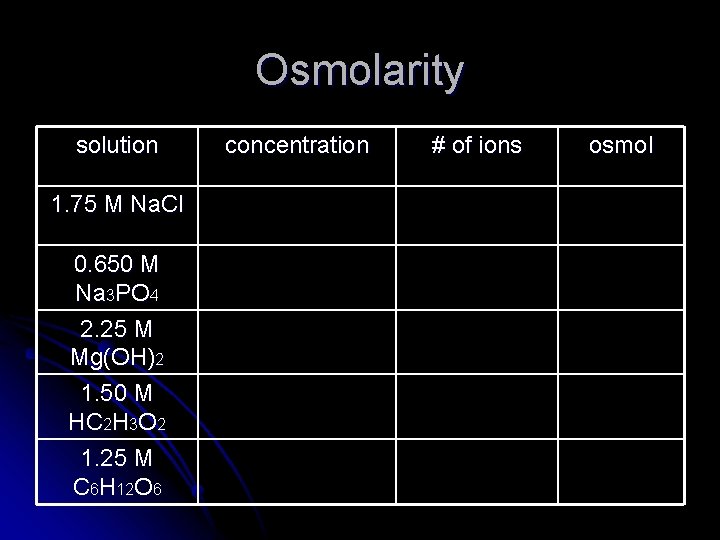
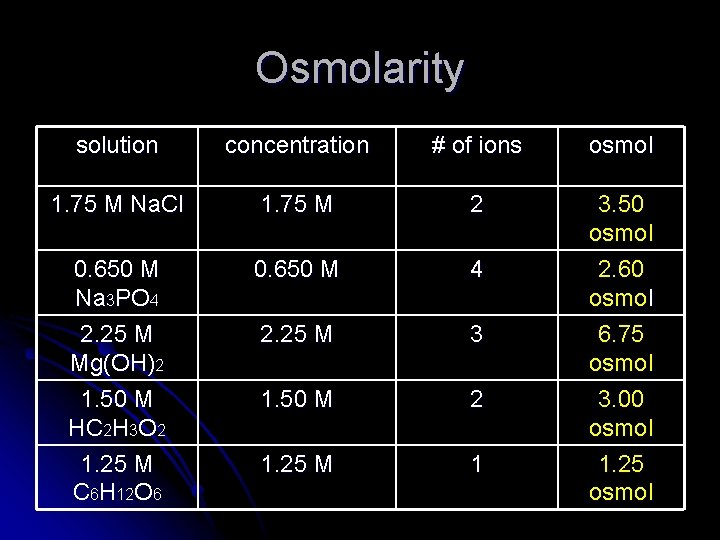
When talking about colligative properties, it is convenient to talk about osmolarity (osmol). The solution with the highest osmolarity will have the most number of particles in solution. Osmol= (# ions/molc) x Molarity For ionic compounds, count the total number of ions in a molecule. For a covalent compound, the number of ions per molecule will be 1.

Osmolarity solution 1. 75 M Na. Cl 0. 650 M Na 3 PO 4 2. 25 M Mg(OH)2 1. 50 M HC 2 H 3 O 2 1. 25 M C 6 H 12 O 6 concentration # of ions osmol

Osmolarity solution concentration # of ions osmol 1. 75 M Na. Cl 1. 75 M 2 0. 650 M Na 3 PO 4 2. 25 M Mg(OH)2 1. 50 M HC 2 H 3 O 2 1. 25 M C 6 H 12 O 6 0. 650 M 4 2. 25 M 3 1. 50 M 2 1. 25 M 1 3. 50 osmol 2. 60 osmol 6. 75 osmol 3. 00 osmol 1. 25 osmol